Efficacy and safety of vorapaxar as approved for clinical use in the United States
- PMID: 25792124
- PMCID: PMC4392433
- DOI: 10.1161/JAHA.114.001505
Efficacy and safety of vorapaxar as approved for clinical use in the United States
Erratum in
-
Efficacy and safety of vorapaxar as approved for clinical use in the United States.J Am Heart Assoc. 2015 Apr 27;4(4):e000633. doi: 10.1161/JAHA.115.000633. J Am Heart Assoc. 2015. PMID: 25917442 Free PMC article. No abstract available.
Abstract
Background: Vorapaxar is a protease-activated receptor-1 antagonist approved by the U.S. Food and Drug Administration (FDA) for the reduction of thrombotic cardiovascular (CV) events in patients with a history of myocardial infarction (MI) and peripheral artery disease (PAD), without a previous stroke or transient ischemic attack (TIA).
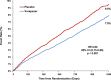
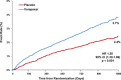
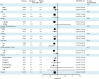
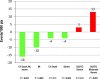
Methods and results: We examined the efficacy and safety of vorapaxar in the intended use population, considering 20,170 patients randomized in the multinational, double-blinded, placebo-controlled TRA 2°P-TIMI 50 trial. Of these, 16,897 qualified with a history of MI in the prior 2 weeks to 1 year and 3273 with PAD. At baseline 97% of the patients were treated with aspirin, 71% with a thienopyridine, and 93% a statin. At 3 years, the endpoint of CV death, MI, or stroke was significantly reduced with vorapaxar compared with placebo (7.9% versus 9.5%, HR, 0.80; 95% CI 0.73 to 0.89; P<0.001). Vorapaxar also significantly reduced the composite of CV death, MI, stroke, and urgent coronary revascularization (10.1% versus 11.8%, HR, 0.83; 95% CI 0.76 to 0.90; P<0.001), as well as the rate of CV death or MI (P<0.001). The safety endpoint of GUSTO moderate or severe bleeding, was increased in the vorapaxar group (3.7 versus 2.4, HR, 1.55; 95% CI 1.30 to 1.86, P<0.001). Intracranial bleeding (ICH) was 0.6% versus 0.4%, P=0.10 with vorapaxar versus placebo, with fatal bleeding 0.2% versus 0.2%; P=0.70.
Conclusions: In patients with prior MI or PAD who have not had a previous stroke or TIA, vorapaxar added to standard therapy is effective for long-term secondary prevention of thrombotic CV events, while increasing moderate or severe bleeding.
Clinical trial registration: URL: clinicaltrials.gov Unique Identifier: NCT00526474.
Keywords: antiplatelet therapy; atherosclerosis; myocardial infarction; peripheral arterial disease; secondary prevention; vorapaxar.
© 2015 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures



Comment in
-
Balancing the risks and benefits of long-term antiplatelet therapies for cardiovascular disease: clinical, research, and regulatory implications.J Am Heart Assoc. 2015 Mar 19;4(3):e001897. doi: 10.1161/JAHA.115.001897. J Am Heart Assoc. 2015. PMID: 25792126 Free PMC article. No abstract available.
References
-
- Bonaca MP, Morrow DA. SCH 530348: a novel oral thrombin receptor antagonist. Future Cardiol. 2009; 5:435-442. - PubMed
-
- Morrow DA, Braunwald E, Bonaca MP, Ameriso SF, Dalby AJ, Fish MP, Fox KA, Lipka LJ, Liu X, Nicolau JC, Ophuis AJ, Paolasso E, Scirica BM, Spinar J, Theroux P, Wiviott SD, Strony J, Murphy SATRA 2P–TIMI 50 Steering Committee and Investigators. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012; 366:1404-1413. - PubMed
-
- Scirica BM, Bonaca MP, Braunwald E, De Ferrari GM, Isaza D, Lewis BS, Mehrhof F, Merlini PA, Murphy SA, Sabatine MS, Tendera M, Van de Werf F, Wicox R, Morrow DATRA 2°P–TIMI 50 Steering Committee Investigators. Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2°P‐TIMI 50 trial. Lancet. 2012; 380:1317-1324. - PubMed
-
- Bonaca MP, Scirica BM, Creager MA, Olin J, Bounameaux H, Dellborg M, Lamp JM, Murphy SA, Braunwald EB, Morrow DA. Vorapaxar in patients with peripheral artery disease: results from TRA 2°P‐TIMI 50. Circulation. 2013; 127:1522-1529. - PubMed
-
- Morrow DA, Alberts MJ, Mohr JP, Amerisio SF, Bonaca MP, Goto S, Hankey GJ, Murphy SA, Scirica BM, Braunwald ETRA 2P–TIMI 50 Steering Committee and Investigators. Efficacy and safety of vorapaxar in patients with prior ischemic stroke. Stroke. 2013; 44:691-698. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

