Antiviral and Immunoregulatory Effects of Indoleamine-2,3-Dioxygenase in Hepatitis C Virus Infection
- PMID: 25792183
- PMCID: PMC4553081
- DOI: 10.1159/000375161
Antiviral and Immunoregulatory Effects of Indoleamine-2,3-Dioxygenase in Hepatitis C Virus Infection
Abstract
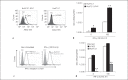
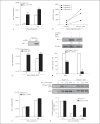
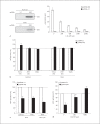
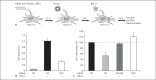
In patients with hepatitis C virus (HCV) infection, enhanced activity of indoleamine-2,3-dioxygenase 1 (IDO) has been reported. IDO - a tryptophan-catabolizing enzyme - has been considered as both an innate defence mechanism and an important regulator of the immune response. The molecular mechanism of IDO induction in HCV infection and its role in the antiviral immune response remain unknown. Using primary human hepatocytes, we show that HCV infection stimulates IDO expression. IDO gene induction was transient and coincided with the expression of types I and III interferons (IFNs) and IFN-stimulated genes in HCV-infected hepatocytes. Overexpression of hepatic IDO prior to HCV infection markedly impaired HCV replication in hepatocytes, suggesting that IDO limits the spread of HCV within the liver. siRNA-mediated IDO knock-down revealed that IDO functions as an IFN-mediated anti-HCV effector. Hepatic IDO was most potently induced by IFN-x03B3;, and ongoing HCV replication could significantly upregulate IDO expression. IRF1 (IFN-regulatory factor 1) and STAT1 (signal transducer and activator of transcription 1) regulated hepatic IDO expression. Hepatic IDO expression also had a significant inhibitory effect on CD4+ T-cell proliferation. Our data suggest that hepatic IDO plays a dual role during HCV infection by slowing down viral replication and also regulating host immune responses.
© 2015 S. Karger AG, Basel.
Figures



Similar articles
-
DDX60L Is an Interferon-Stimulated Gene Product Restricting Hepatitis C Virus Replication in Cell Culture.J Virol. 2015 Oct;89(20):10548-68. doi: 10.1128/JVI.01297-15. Epub 2015 Aug 12. J Virol. 2015. PMID: 26269178 Free PMC article.
-
Hepatitis C virus replication in mouse cells is restricted by IFN-dependent and -independent mechanisms.Gastroenterology. 2013 Dec;145(6):1414-23.e1. doi: 10.1053/j.gastro.2013.08.037. Epub 2013 Aug 21. Gastroenterology. 2013. PMID: 23973921
-
HCV infection induces a unique hepatic innate immune response associated with robust production of type III interferons.Gastroenterology. 2012 Apr;142(4):978-88. doi: 10.1053/j.gastro.2011.12.055. Epub 2012 Jan 13. Gastroenterology. 2012. PMID: 22248663 Free PMC article.
-
Multi-step regulation of interferon induction by hepatitis C virus.Arch Immunol Ther Exp (Warsz). 2013 Apr;61(2):127-38. doi: 10.1007/s00005-012-0214-x. Epub 2013 Jan 5. Arch Immunol Ther Exp (Warsz). 2013. PMID: 23292079 Review.
-
Interferon Response in Hepatitis C Virus-Infected Hepatocytes: Issues to Consider in the Era of Direct-Acting Antivirals.Int J Mol Sci. 2020 Apr 8;21(7):2583. doi: 10.3390/ijms21072583. Int J Mol Sci. 2020. PMID: 32276399 Free PMC article. Review.
Cited by
-
Short- and Long-Term Immunological Responses in Chronic HCV/HIV Co-Infected Compared to HCV Mono-Infected Patients after DAA Therapy.Pathogens. 2021 Nov 15;10(11):1488. doi: 10.3390/pathogens10111488. Pathogens. 2021. PMID: 34832642 Free PMC article.
-
On PAMPs and DAMPs.J Innate Immun. 2016;8(5):427-8. doi: 10.1159/000448437. Epub 2016 Aug 13. J Innate Immun. 2016. PMID: 27522675 Free PMC article. No abstract available.
-
Indoleamine 2,3-dioxygenase: As a potential prognostic marker and immunotherapeutic target for hepatocellular carcinoma.World J Gastroenterol. 2017 Apr 7;23(13):2286-2293. doi: 10.3748/wjg.v23.i13.2286. World J Gastroenterol. 2017. PMID: 28428708 Free PMC article. Review.
-
Indoleamine 2,3-Dioxygenase Is Involved in Interferon Gamma's Anti-BKPyV Activity in Renal Cells.Viruses. 2020 Aug 7;12(8):865. doi: 10.3390/v12080865. Viruses. 2020. PMID: 32784805 Free PMC article.
-
Modeling the Immune Response for Pathogenic and Nonpathogenic Orthohantavirus Infections in Human Lung Microvasculature Endothelial Cells.Viruses. 2023 Aug 24;15(9):1806. doi: 10.3390/v15091806. Viruses. 2023. PMID: 37766212 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

