Prevention of bone loss with risedronate in breast cancer survivors: a randomized, controlled clinical trial
- PMID: 25792492
- PMCID: PMC4766869
- DOI: 10.1007/s00198-015-3100-7
Prevention of bone loss with risedronate in breast cancer survivors: a randomized, controlled clinical trial
Abstract
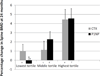
In postmenopausal women with low bone mass and hormone-receptor-positive breast cancer on an aromatase inhibitor, risedronate maintained skeletal health assessed by bone density and turnover markers. Women with the greatest decreases in bone turnover markers at 12 months had the greatest increases in bone density at 24 months.
Introduction: Aromatase inhibitors (AIs), adjuvant endocrine therapy for postmenopausal women with hormone-receptor-positive breast cancer, are associated with bone loss and fractures. Our objectives were to determine if (1) oral bisphosphonate therapy can prevent bone loss in women on an AI and (2) early changes in bone turnover markers (BTM) can predict later changes in bone mineral density (BMD).
Methods: We conducted a 2-year double-blind, placebo-controlled, randomized trial in 109 postmenopausal women with low bone mass on an AI (anastrozole, letrozole, or exemestane) for hormone-receptor-positive breast cancer. Participants were randomized to once weekly risedronate 35 mg or placebo, and all received calcium plus vitamin D. The main outcome measures included BMD, BTM [carboxy-terminal collagen crosslinks (CTX) and N-terminal propeptide of type 1 procollagen (P1NP)], and safety.
Results: Eighty-seven percent completed 24 months. BMD increased more in the active treatment group compared to placebo with an adjusted difference at 24 months of 3.9 ± 0.7 percentage points at the spine and 3.2 ± 0.5 percentage points at the hip (both p < 0.05). The adjusted difference between the active treatment and placebo groups were 0.09 ± 0.04 nmol/LBCE for CTX and 23.3 ± 4.8 μg/mL for P1NP (both p < 0.05). Women with greater 12-month decreases in CTX and P1NP in the active treatment group had a greater 24-month increase in spinal BMD (p < 0.05). The oral therapy was safe and well tolerated.
Conclusion: In postmenopausal women with low bone mass and breast cancer on an AI, the oral bisphosphonate risedronate maintained skeletal health.
Keywords: Antiresorptives; Biochemical markers of bone turnover; Clinical trials; DXA.
Conflict of interest statement
Figures


References
-
- Becker T, Lipscombe L, Narod S, Simmons C, Anderson GM, Rochon PA. Systematic review of bone health in older women treated with aromatase inhibitors for early-stage breast cancer. J Am Geriatr Soc. 2012;60(9):1761–1767. Epub 2012/09/19. PubMed PMID: 22985145. - PubMed
-
- Greenspan SL, Brufsky A, Lembersky BC, Bhattacharya R, Vujevich KT, Perera S, et al. Risedronate Prevents Bone Loss in Breast Cancer Survivors: a 2 Year, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J Clin Oncol. 2008;26(16):2644–2652. PubMed PMID: 18427147; PubMed Central PMCID: PMCPmc3992822. - PMC - PubMed
-
- Brufsky AM, Harker WG, Beck JT, Bosserman L, Vogel C, Seidler C, et al. Final 5-year results of Z-FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer. 2012;118(5):1192–1201. Epub 2011/10/12. PubMed PMID: 21987386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

