Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease
- PMID: 25792665
- PMCID: PMC4478793
- DOI: 10.1096/fj.14-268276
Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease
Abstract
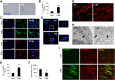
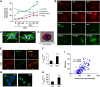
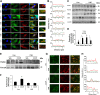
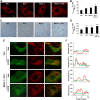
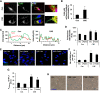
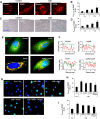
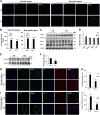
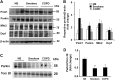
Cigarette smoke (CS)-induced cellular senescence is involved in the pathogenesis of chronic obstructive pulmonary disease (COPD). The molecular mechanism by which CS induces cellular senescence is unknown. Here, we show that CS stress (exposure of primary lung cells to CS extract 0.2-0.75% with a half-maximal inhibitory concentration of ∼0.5%) led to impaired mitophagy and perinuclear accumulation of damaged mitochondria associated with cellular senescence in both human lung fibroblasts and small airway epithelial cells (SAECs). Impaired mitophagy was attributed to reduced Parkin translocation to damaged mitochondria, which was due to CS-induced cytoplasmic p53 accumulation and its interaction with Parkin. Impaired Parkin translocation to damaged mitochondria was also observed in mouse lungs with emphysema (6 months CS exposure, 100 mg TPM/m(3)) as well as in lungs of chronic smokers and patients with COPD. Primary SAECs from patients with COPD also exhibited impaired mitophagy and increased cellular senescence via suborganellar signaling. Mitochondria-targeted antioxidant (Mito-Tempo) restored impaired mitophagy, decreased mitochondrial mass accumulation, and delayed cellular senescence in Parkin-overexpressing cells. In conclusion, defective mitophagy leads to CS stress-induced lung cellular senescence, and restoring mitophagy delays cellular senescence, which provides a promising therapeutic intervention in chronic airway diseases.
Keywords: DNA damage; Parkin; Pink1; perinuclear mitochondrial clustering; reactive oxygen species.
© FASEB.
Figures


References
-
- Nyunoya T., Monick M. M., Klingelhutz A. L., Glaser H., Cagley J. R., Brown C. O., Matsumoto E., Aykin-Burns N., Spitz D. R., Oshima J., Hunninghake G. W. (2009) Cigarette smoke induces cellular senescence via Werner’s syndrome protein down-regulation. Am. J. Respir. Crit. Care Med. 179, 279–287 - PMC - PubMed
-
- Hara H., Araya J., Takasaka N., Fujii S., Kojima J., Yumino Y., Shimizu K., Ishikawa T., Numata T., Kawaishi M., Saito K., Hirano J., Odaka M., Morikawa T., Hano H., Nakayama K., Kuwano K. (2012) Involvement of creatine kinase B in cigarette smoke-induced bronchial epithelial cell senescence. Am. J. Respir. Cell Mol. Biol. 46, 306–312 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

