A randomised, double-blind trial comparing budesonide formulations and dosages for short-term treatment of eosinophilic oesophagitis
- PMID: 25792708
- PMCID: PMC4789829
- DOI: 10.1136/gutjnl-2014-308815
A randomised, double-blind trial comparing budesonide formulations and dosages for short-term treatment of eosinophilic oesophagitis
Abstract
Objective: To investigate the efficacy and safety of two different budesonide formulations (effervescent tablet for orodispersible use (BET) and viscous suspension (BVS)) with different daily dosages for short-term treatment of eosinophilic oesophagitis (EoE).
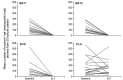
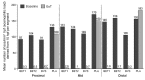
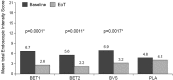
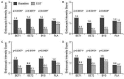
Design: Adults with active EoE (n=76) randomly received 14 days' treatment with either BET 2×1 mg/day (BET1, n=19) or BET 2×2 mg/day (BET2, n=19), or BVS 2×5 mL (0.4 mg/mL)/day (BVS, n=19) or placebo (n=19) in a double-blind, double-dummy fashion, with a 2-week follow-up. Primary end point was histological remission (mean of <16 eosinophils/mm(2 )hpf). Secondary end points included endoscopy score, dysphagia score, drug safety and patient's preference for drug formulation.
Results: Histological remission occurred in 100%, 94.7% and 94.7% of budesonide (BET1, BET2, BVS, respectively) and in 0% of placebo recipients (p<0.0001). The improvement in total endoscopic intensity score was significantly higher in the three budesonide groups compared with placebo. Dysphagia improved in all groups at the end of treatment; however, improvement of dysphagia persisted only in those treated with BET1 (p=0.0196 vs placebo). There were no serious adverse events. Local fungal infection (stained fungi) occurred in two patients of each budesonide group (10.5%). The effervescent tablet was preferred by 80% of patients.
Conclusions: BET or BVS was highly effective and safe for short-term treatment of EoE. The 1 mg (twice daily) dosage was equally effective as the 2 mg twice daily dosage. The majority of patients preferred the effervescent tablet formulation.
Clinicaltrialsgov number: NCT02280616; EudraCT number, 2009-016692-29.
Keywords: DYSPHAGIA; OESOPHAGITIS.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
