Structural basis for competitive inhibition of 3,4-dihydroxy-2-butanone-4-phosphate synthase from Vibrio cholerae
- PMID: 25792735
- PMCID: PMC4416836
- DOI: 10.1074/jbc.M114.611830
Structural basis for competitive inhibition of 3,4-dihydroxy-2-butanone-4-phosphate synthase from Vibrio cholerae
Abstract
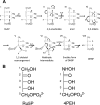
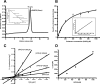
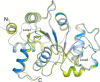
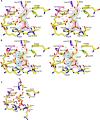
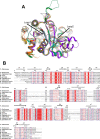
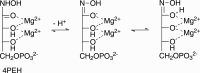
The riboflavin biosynthesis pathway has been shown to be essential in many pathogens and is absent in humans. Therefore, enzymes involved in riboflavin synthesis are considered as potential antibacterial drug targets. The enzyme 3,4-dihydroxy-2-butanone-4-phosphate synthase (DHBPS) catalyzes one of the two committed steps in the riboflavin pathway and converts d-ribulose 5-phosphate (Ru5P) to l-3,4-dihydroxy-2-butanone 4-phosphate and formate. Moreover, DHBPS is shown to be indispensable for Mycobacterium, Salmonella, and Helicobacter species. Despite the essentiality of this enzyme in bacteria, no inhibitor has been identified hitherto. Here, we describe kinetic and crystal structure characterization of DHBPS from Vibrio cholerae (vDHBPS) with a competitive inhibitor 4-phospho-d-erythronohydroxamic acid (4PEH) at 1.86-Å resolution. In addition, we also report the structural characterization of vDHBPS in its apo form and in complex with its substrate and substrate plus metal ions at 1.96-, 1.59-, and 2.04-Å resolution, respectively. Comparison of these crystal structures suggests that 4PEH inhibits the catalytic activity of DHBPS as it is unable to form a proposed intermediate that is crucial for DHBPS activity. Furthermore, vDHBPS structures complexed with substrate and metal ions reveal that, unlike Candida albicans, binding of substrate to vDHBPS induces a conformational change from an open to closed conformation. Interestingly, the position of second metal ion, which is different from that of Methanococcus jannaschii, strongly supports an active role in the catalytic mechanism. Thus, the kinetic and structural characterization of vDHBPS reveals the molecular mechanism of inhibition shown by 4PEH and that it can be explored further for designing novel antibiotics.
Keywords: Crystal Structure; DHBPS; Enzyme Inhibitor; Enzyme Kinetics; Enzyme Mechanism; Riboflavin; Ribulose 5-Phosphate; ribB.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


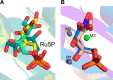

References
-
- Bacher A. (1991) in Chemistry and Biochemistry of Flavoenzymes (Müller F., ed) Vol. I, pp. 349–370, CRC Press, Boca Raton, FL
-
- Cole S. T., Eiglmeier K., Parkhill J., James K. D., Thomson N. R., Wheeler P. R., Honoré N., Garnier T., Churcher C., Harris D., Mungall K., Basham D., Brown D., Chillingworth T., Connor R., Davies R. M., Devlin K., Duthoy S., Feltwell T., Fraser A., Hamlin N., Holroyd S., Hornsby T., Jagels K., Lacroix C., Maclean J., Moule S., Murphy L., Oliver K., Quail M. A., Rajandream M. A., Rutherford K. M., Rutter S., Seeger K., Simon S., Simmonds M., Skelton J., Squares R., Squares S., Stevens K., Taylor K., Whitehead S., Woodward J. R., Barrell B. G. (2001) Massive gene decay in the leprosy bacillus. Nature 409, 1007–1011 - PubMed
-
- Gerdes S. Y., Scholle M. D., D'Souza M., Bernal A., Baev M. V., Farrell M., Kurnasov O. V., Daugherty M. D., Mseeh F., Polanuyer B. M., Campbell J. W., Anantha S., Shatalin K. Y., Chowdhury S. A., Fonstein M. Y., Osterman A. L. (2002) From genetic footprinting to antimicrobial drug targets: examples in cofactor biosynthetic pathways. J. Bacteriol. 184, 4555–4572 - PMC - PubMed
-
- Sassetti C. M., Boyd D. H., Rubin E. J. (2003) Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48, 77–84 - PubMed
-
- Bacher A., Eberhardt S., Richter G. (1996) in Escherichia coli and Salmonella: Cellular and Molecular Biology (Neidhardt F. C., Ingraham J. L., Low K. B., Magasanik B., Schaechter M., Umbarger H. E., eds), Vol. 1, 2nd Ed., pp. 657–664, ASM Press, Washington, D. C.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

