Single dose pharmacokinetics of the novel transdermal donepezil patch in healthy volunteers
- PMID: 25792802
- PMCID: PMC4362658
- DOI: 10.2147/DDDT.S78555
Single dose pharmacokinetics of the novel transdermal donepezil patch in healthy volunteers
Abstract
Background: Donepezil is an acetylcholinesterase inhibitor indicated for Alzheimer's disease. The aim of this randomized, single-blind, placebo-controlled, single-dose, dose-escalation study was to investigate the safety, tolerability, and pharmacokinetics of the donepezil patch in healthy male subjects.
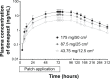
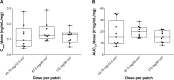
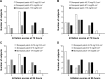
Methods: Each healthy male subject received a single transdermal donepezil patch (72 hours patch-on periods) of 43.75 mg/12.5 cm(2), 87.5 mg/25 cm(2), or 175 mg/50 cm(2). Serial blood samples were collected up to 312 hours after patch application. The plasma concentrations of donepezil were determined by using a validated liquid chromatography-tandem mass spectrometry method. Pharmacokinetic parameters were obtained by noncompartmental analysis. Tolerability of the patches and performance of the patches (adhesion, skin irritation, residual donepezil content in the patch) were assessed throughout the study.
Results: The study was completed by 36 healthy subjects. After patch application, the maximal plasma donepezil concentration (Cmax) and the area under the curve (AUC) increased in a dose-proportional manner. Median time to Cmax was ~74-76 hours (~2-4 hours after patch removal), and mean t1/2β was ~63.77-93.07 hours. The average donepezil residue in the patch after 72 hours was ~73.9%-86.7% of the loading dose. There were neither serious adverse events nor adverse events that lead to discontinuation. Skin adhesion of the patch was good in 97.2% of the subjects. All skin irritations after patch removal were mild and were resolved during the study period.
Conclusion: The donepezil patch appeared to be generally well tolerated and adhesive. Pharmacokinetic analysis of the donepezil patch demonstrated linear kinetics.
Keywords: donepezil; healthy subjects; pharmacokinetics; transdermal patch.
Figures
Similar articles
-
Therapeutic dosage assessment based on population pharmacokinetics of a novel single-dose transdermal donepezil patch in healthy volunteers.Eur J Clin Pharmacol. 2015 Aug;71(8):967-77. doi: 10.1007/s00228-015-1875-2. Epub 2015 May 28. Eur J Clin Pharmacol. 2015. PMID: 26014587 Clinical Trial.
-
Pharmacokinetic comparison of orally disintegrating and conventional donepezil formulations in healthy Korean male subjects: a single-dose, randomized, open-label, 2-sequence, 2-period crossover study.Clin Ther. 2011 Jul;33(7):965-72. doi: 10.1016/j.clinthera.2011.06.003. Epub 2011 Jul 2. Clin Ther. 2011. PMID: 21723605 Clinical Trial.
-
Concurrent administration of donepezil HCl and levodopa/carbidopa in patients with Parkinson's disease: assessment of pharmacokinetic changes and safety following multiple oral doses.Br J Clin Pharmacol. 2004 Nov;58 Suppl 1(Suppl 1):41-9. doi: 10.1111/j.1365-2125.2004.01799.x. Br J Clin Pharmacol. 2004. PMID: 15496222 Free PMC article. Clinical Trial.
-
Bioequivalence study of two different tablet formulations of donepezil using truncated areas under the curve. A single-center, single-dose, randomized, open-label, 2-way crossover study under fasting conditions.Arzneimittelforschung. 2010;60(3):116-23. doi: 10.1055/s-0031-1296259. Arzneimittelforschung. 2010. PMID: 20422942 Clinical Trial.
-
Rapid and sensitive determination of donepezil in human plasma by liquid chromatography/tandem mass spectrometry: application to a pharmacokinetic study.Rapid Commun Mass Spectrom. 2006;20(21):3193-8. doi: 10.1002/rcm.2718. Rapid Commun Mass Spectrom. 2006. PMID: 17016805
Cited by
-
A Multinational, Multicenter, Randomized, Double-Blind, Active Comparator, Phase III Clinical Trial to Evaluate the Efficacy and Safety of Donepezil Transdermal Patch in Patients With Alzheimer's Disease.J Clin Neurol. 2022 Jul;18(4):428-436. doi: 10.3988/jcn.2022.18.4.428. J Clin Neurol. 2022. PMID: 35796268 Free PMC article.
-
Pharmacokinetic Evaluation by Modeling and Simulation Analysis of a Donepezil Patch Formulation in Healthy Male Volunteers.Drug Des Devel Ther. 2020 May 5;14:1729-1737. doi: 10.2147/DDDT.S244957. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32440098 Free PMC article.
-
Therapeutic dosage assessment based on population pharmacokinetics of a novel single-dose transdermal donepezil patch in healthy volunteers.Eur J Clin Pharmacol. 2015 Aug;71(8):967-77. doi: 10.1007/s00228-015-1875-2. Epub 2015 May 28. Eur J Clin Pharmacol. 2015. PMID: 26014587 Clinical Trial.
-
Optimized method development and validation for determining donepezil in rat plasma: A liquid-liquid extraction, LC-MS/MS, and design of experiments approach.PLoS One. 2024 Sep 6;19(9):e0309802. doi: 10.1371/journal.pone.0309802. eCollection 2024. PLoS One. 2024. PMID: 39240870 Free PMC article.
-
Translation from Preclinical Research to Clinical Trials: Transdermal Drug Delivery for Neurodegenerative and Mental Disorders.Pharm Res. 2024 Jun;41(6):1045-1092. doi: 10.1007/s11095-024-03718-x. Epub 2024 Jun 11. Pharm Res. 2024. PMID: 38862719 Review.
References
-
- Small GW, Rabins PV, Barry PP, et al. Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. JAMA. 1997;278(16):1363–1371. - PubMed
-
- Berchtold NC, Cotman CW. Evolution in the conceptualization of dementia and Alzheimer’s disease: Greco-Roman period to the 1960s. Neurobiol Aging. 1998;19(3):173–189. - PubMed
-
- Alzheimer’s Disease International . World Alzheimer Report 2012. Overcoming the Stigma of Dementia. London: Alzheimer’s Disease International; 2012. [Accessed November 11, 2014]. Available from: http://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

