Cryo-ablation improves anti-tumor immunity through recovering tumor educated dendritic cells in tumor-draining lymph nodes
- PMID: 25792805
- PMCID: PMC4362656
- DOI: 10.2147/DDDT.S76592
Cryo-ablation improves anti-tumor immunity through recovering tumor educated dendritic cells in tumor-draining lymph nodes
Abstract
Background: In addition to minimally invasive destruction of tumors, cryo-ablation of tumors to some extent modulated anti-tumor immunity. Cryo-ablated tumors in glioma mice models induced anti-tumor cellular immunologic response which increases the percentage of CD3(+) and CD4(+)T cells in blood as well as natural killer cells. As a crucial role in triggering anti-tumor immunity, dendritic cells (DCs) were educated by tumors to adopt a tolerance phenotype which helps the tumor escape from immune monitoring. This study aims to study whether cryo-ablation could influence the tolerogenic DCs, and influence anti-tumor immunity in tumor-draining lymph nodes (TDLNs).
Methods: Using the GL261 subcutaneous glioma mouse model, we created a tumor bearing group, cryo-ablation group, and surgery group. We analyzed alteration in phenotype and function of tolerogenic DCs, and evaluated the factors of anti-tumor immunity inhibition.
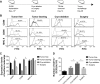
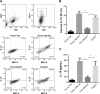
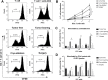
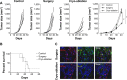
Results: DCs in TDLNs in GL261 subcutaneous glioma mouse model expressed tolerogenic phenotype. In contrast to surgery, cryo-ablation improved the quantity and quality of these tolerogenic DCs. Moreover, the DCs decreased the expression of intracellular interleukin-10 (IL-10) and extra-cellular IL-10. In vitro, DCs from the cryo-ablation group recovered their specific function and induced potent anti-tumor immunity through triggering T cells. In vivo, cryo-ablation showed weak anti-tumor immunity, only inhibiting the growth of rechallenged tumors. But many IL-10-low DCs, rather than IL-10-high DCs, infiltrated the tumors. More importantly, Tregs inhibited the performance of these DCs; and depletion of Tregs greatly improved anti-tumor immunity in vivo.
Conclusion: Cryo-ablation could recover function of tumor induced tolerogenic DCs in vitro; and depletion of Tregs could improve this anti-tumor effect in vivo. The Tregs/CD4(+)T and Tregs/CD25(+)T cells in TDLNs inhibit DCs' activity and function.
Keywords: anti-tumor immunity; cryo-ablation; dendritic cells; glioma; tumor-draining lymph nodes.
Figures

References
-
- Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. - PubMed
-
- Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179(2):977–983. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

