A review of infections in patients with left ventricular assist devices: prevention, diagnosis and management
- PMID: 25793027
- PMCID: PMC4362062
- DOI: 10.14797/mdcj-11-1-28
A review of infections in patients with left ventricular assist devices: prevention, diagnosis and management
Abstract
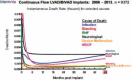
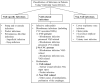
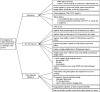
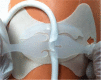
Since the advent of ventricular assist devices with smaller configurations and continuous-flow technology, survival rates for patients with end-stage heart failure have dramatically improved. While the burden of infectious complications is decreased in patients on continuous-flow ventricular assist devices compared to bulkier pulsatile-flow devices, infection remains one of the most common causes of morbidity and mortality. The majority of infections occur at the driveline exit site, beginning with a disruption or trauma to the barrier between the skin and driveline and sometimes spreading deeper. Once infections develop, they can be difficult to eradicate. Depending on the degree of infection, treatment options may include local wound care, antibiotics, or surgical treatment. Preventive strategies and careful surveillance are crucial to improve patient outcomes.
Keywords: driveline; infection; left ventricular assist device.
Figures


References
-
- Slaughter MS, Rogers JG, Milano CA et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009 Dec 3;361(23):2241–51. - PubMed
-
- Kirklin JK, Naftel DC, Kormos RL et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2014 Jan;33(1):12–22. - PubMed
-
- Patel CB, Cowger JA, Zuckermann A. A contemporary review of mechanical circulatory support. J Heart Lung Transplant. 2014 Jul;33(7):667–74. - PubMed
-
- Smedira NG, Hoercher KJ, Lima B et al. Unplanned hospital readmissions after HeartMate II implantation: frequency, risk factors, and impact on resource use and survival. JACC Heart Fail. 2013 Feb;1(1):31–9. - PubMed
-
- Goldstein DJ, Naftel D, Holman W et al. Continuous-flow devices and percutaneous site infections: clinical outcomes. J Heart Lung Transplant. 2012 Nov;31(11):1151–7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

