Dimethyl fumarate and monoethyl fumarate exhibit differential effects on KEAP1, NRF2 activation, and glutathione depletion in vitro
- PMID: 25793262
- PMCID: PMC4368598
- DOI: 10.1371/journal.pone.0120254
Dimethyl fumarate and monoethyl fumarate exhibit differential effects on KEAP1, NRF2 activation, and glutathione depletion in vitro
Abstract
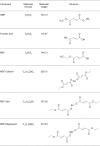
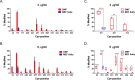
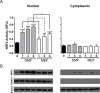
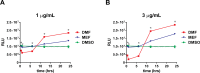
Delayed-release dimethyl fumarate (also known as gastro-resistant dimethyl fumarate), an oral therapeutic containing dimethyl fumarate (DMF) as the active ingredient, is currently approved for the treatment of relapsing multiple sclerosis. DMF is also a component in a distinct mixture product with 3 different salts of monoethyl fumarate (MEF), which is marketed for the treatment of psoriasis. Previous studies have provided insight into the pharmacologic properties of DMF, including modulation of kelch-like ECH-associated protein 1 (KEAP1), activation of the nuclear factor (erythroid-derived 2)-like 2 (NRF2) pathway, and glutathione (GSH) modulation; however, those of MEF remain largely unexplored. Therefore, the aim of this study was to evaluate the in vitro effects of DMF and MEF on KEAP1 modification, activation of the NRF2 pathway, and GSH conjugation. Using mass spectrometry, DMF treatment resulted in a robust modification of specific cysteine residues on KEAP1. In comparison, the overall degree of KEAP1 modification following MEF treatment was significantly less or undetectable. Consistent with KEAP1 cysteine modification, DMF treatment resulted in nuclear translocation of NRF2 and a robust transcriptional response in treated cells, as did MEF; however, the responses to MEF were of a lower magnitude or distinct compared to DMF. DMF was also shown to produce an acute concentration-dependent depletion of GSH; however, GSH levels eventually recovered and rose above baseline by 24 hours. In contrast, MEF did not cause acute reductions in GSH, but did produce an increase by 24 hours. Overall, these studies demonstrate that DMF and MEF are both pharmacologically active, but have differing degrees of activity as well as unique actions. These differences would be expected to result in divergent effects on downstream biology.
Conflict of interest statement
Figures


References
-
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343: 938–952. - PubMed
-
- Fox RJ, Bethoux F, Goldman MD, Cohen JA. Multiple sclerosis: advances in understanding, diagnosing, and treating the underlying disease. Cleve Clin J Med. 2006;73: 91–102. - PubMed
-
- Freedman MS. Disease-modifying drugs for multiple sclerosis: current and future aspects. Expert Opin Pharmacother. 2006;Suppl 1: S1–9. - PubMed
-
- Gilgun-Sherki Y, Melamed E, Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol. 2004;25: 261–268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

