Microvesicles derived from human umbilical cord mesenchymal stem cells facilitate tubular epithelial cell dedifferentiation and growth via hepatocyte growth factor induction
- PMID: 25793303
- PMCID: PMC4368636
- DOI: 10.1371/journal.pone.0121534
Microvesicles derived from human umbilical cord mesenchymal stem cells facilitate tubular epithelial cell dedifferentiation and growth via hepatocyte growth factor induction
Abstract
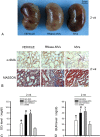
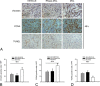
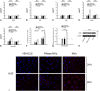
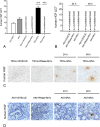
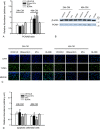
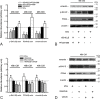
During acute kidney injury (AKI), tubular cell dedifferentiation initiates cell regeneration; hepatocyte growth factor (HGF) is involved in modulating cell dedifferentiation. Mesenchymal stem cell (MSC)-derived microvesicles (MVs) deliver RNA into injured tubular cells and alter their gene expression, thus regenerating these cells. We boldly speculated that MVs might induce HGF synthesis via RNA transfer, thereby facilitating tubular cell dedifferentiation and regeneration. In a rat model of unilateral AKI, the administration of MVs promoted kidney recovery. One of the mechanisms of action is the acceleration of tubular cell dedifferentiation and growth. Both in vivo and in vitro, rat HGF expression in damaged rat tubular cells was greatly enhanced by MV treatment. In addition, human HGF mRNA present in MVs was delivered into rat tubular cells and translated into the HGF protein as another mechanism of HGF induction. RNase treatment abrogated all MV effects. In the in vitro experimental setting, the conditioned medium of MV-treated injured tubular cells, which contains a higher concentration of HGF, strongly stimulated cell dedifferentiation and growth, as well as Erk1/2 signaling activation. Intriguingly, these effects were completely abrogated by either c-Met inhibitor or MEK inhibitor, suggesting that HGF induction is a crucial contributor to the acceleration of cell dedifferentiation and growth. All these findings indicate that MV-induced HGF synthesis in damaged tubular cells via RNA transfer facilitates cell dedifferentiation and growth, which are important regenerative mechanisms.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

