The interaction affinity between vascular cell adhesion molecule-1 (VCAM-1) and very late antigen-4 (VLA-4) analyzed by quantitative FRET
- PMID: 25793408
- PMCID: PMC4368157
- DOI: 10.1371/journal.pone.0121399
The interaction affinity between vascular cell adhesion molecule-1 (VCAM-1) and very late antigen-4 (VLA-4) analyzed by quantitative FRET
Abstract
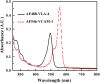
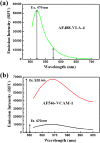
Very late antigen-4 (VLA-4), a member of integrin superfamily, interacts with its major counter ligand vascular cell adhesion molecule-1 (VCAM-1) and plays an important role in leukocyte adhesion to vascular endothelium and immunological synapse formation. However, irregular expressions of these proteins may also lead to several autoimmune diseases and metastasis cancer. Thus, quantifying the interaction affinity of the VCAM-1/VLA-4 interaction is of fundamental importance in further understanding the nature of this interaction and drug discovery. In this study, we report an 'in solution' steady state organic fluorophore based quantitative fluorescence resonance energy transfer (FRET) assay to quantify this interaction in terms of the dissociation constant (Kd). We have used, in our FRET assay, the Alexa Fluor 488-VLA-4 conjugate as the donor, and Alexa Fluor 546-VCAM-1 as the acceptor. From the FRET signal analysis, Kd of this interaction was determined to be 41.82 ± 2.36 nM. To further confirm our estimation, we have employed surface plasmon resonance (SPR) technique to obtain Kd = 39.60 ± 1.78 nM, which is in good agreement with the result obtained by FRET. This is the first reported work which applies organic fluorophore based 'in solution' simple quantitative FRET assay to obtain the dissociation constant of the VCAM-1/VLA-4 interaction, and is also the first quantification of this interaction. Moreover, the value of Kd can serve as an indicator of abnormal protein-protein interactions; hence, this assay can potentially be further developed into a drug screening platform of VLA-4/VCAM-1 as well as other protein-ligand interactions.
Conflict of interest statement
Figures




References
-
- Oppenheimer-Marks N, Davis LS, Bogue DT, Ramberg J, Lipsky PE. Differential utilization of ICAM-1 and VCAM-1 during the adhesion and transendothelial migration of human T lymphocytes. J Immunol. 1991; 147: 2913–2921. - PubMed
-
- Weber C, Springer TA. Interaction of very late antigen-4 with VCAM-1 supports transendothelial chemotaxis of monocytes by facilitating lateral migration. J Immunol. 1998; 161: 6825–6834. - PubMed
-
- Ding Z, Xiong K, Issekutz TB. Chemokines stimulate human T lymphocyte transendothelial migration to utilize VLA-4 in addition to LFA-1. J Leukoc Biol. 2001; 69: 458–466. - PubMed
-
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995; 268: 233–239. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

