Assessment of the quality of reporting of randomised controlled trials in otorhinolaryngologic literature - adherence to the CONSORT statement
- PMID: 25793517
- PMCID: PMC4368673
- DOI: 10.1371/journal.pone.0122328
Assessment of the quality of reporting of randomised controlled trials in otorhinolaryngologic literature - adherence to the CONSORT statement
Abstract
Background: Randomised Controlled Trials (RCTs) are the preferred study design when comparing therapeutical interventions in medicine. To improve clarity, consistency and transparency of reporting RCTs, the Consolidated Standards of Reporting Trials (CONSORT) statement was developed.
Objectives: (1) To assess the quality of reports and abstracts of RCTs in otorhinolaryngologic literature by using CONSORT checklists, (2) to compare the quality of reports and abstracts of otorhinolaryngologic RCTs between the top 5 general medical journals and top 5 otorhinolaryngologic journals, and (3) to formulate recommendations for authors and editors of otorhinolaryngologic ('ENT') journals.
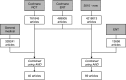
Methods: Based on 2012 ISI Web of Knowledge impact factors, the top 5 general medical and ENT journals were selected. On 25 June 2014, using a highly sensitive Cochrane RCT filter and ENT filter, possibly relevant articles since January 1st, 2010 were retrieved and relevant RCTs were selected. We assessed how many CONSORT items were reported adequately in reports and abstracts and compared the two journal types.
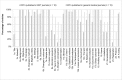
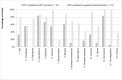
Results: Otorhinolaryngologic RCTs (n = 15) published in general medical journals reported a mean of 92.1% (95% confidence interval: 89.5%-94.7%) of CONSORT items adequately, whereas RCTs (n = 18) published in ENT journals reported a mean of 71.8% (66.7%-76.8%) adequately (p < 0.001). For abstracts, means of 70.0% (63.7%-76.3%) and 32.3% (26.6-38.0%) were found respectively (p < 0.001). Large differences for specific items exist between the two journal types.
Conclusion: The quality of reporting of RCTs in otorhinolaryngologic journals is suboptimal. RCTs published in general medical journals have a higher quality of reporting than RCTs published in ENT journals. We recommend authors to report their trial according to the CONSORT Statement and advise editors to endorse the CONSORT Statement and implement the CONSORT Statement in the editorial process to ensure more adequate reporting of RCTs and their abstracts.
Conflict of interest statement
Figures
References
-
- Agha R, Cooper D, Muir G. The reporting quality of randomised controlled trials in surgery: a systematic review. Int J Surg 2007;5(6): 413–22. - PubMed
-
- Chan AW, Altman DG. Epidemiology and reporting of randomised trials published in PubMed journals. Lancet 2005;365(9465): 1159–62. - PubMed
-
- Chan AW, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials. JAMA 2004;291(20): 2457–65. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

