Synthesis of C-arylnucleoside analogues
- PMID: 25793544
- PMCID: PMC6272657
- DOI: 10.3390/molecules20034967
Synthesis of C-arylnucleoside analogues
Abstract
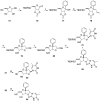
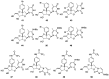
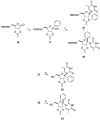
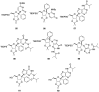
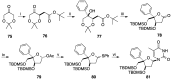
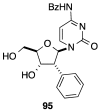
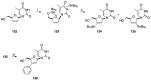
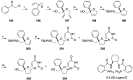
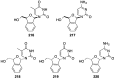
Modified nucleoside analogues are of great biological importance as antiviral and antitumoral agents. There is special interest in the preparation of C-aryl nucleosides with an aromatic ring in different positions of the glycone for their biological activity. Different chemical synthesis strategies for these targets are described in this review.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
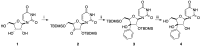












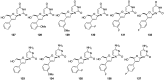



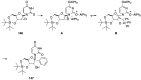

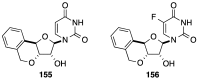



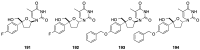


References
-
- Len C., Mackenzie G. Synthesis of 2',3'-didehydro-2',3'-dideoxynucleosides having variations at either or both of the 2'- and 3'-positions. Tetrahedron. 2006;62:9085–9107. doi: 10.1016/j.tet.2006.07.050. - DOI
-
- Len C., Postel D. Synthesis of 2',3'-didehydro-2',3'-dideoxynucleosides via nucleoside route. Curr. Org. Synth. 2006;21:261–281. doi: 10.2174/157017906777934881. - DOI
-
- Len C., Mondon M., Lebreton J. Synthesis of cyclonucleosides having a C-C bridge. Tetrahedron. 2008;64:7453–7475. doi: 10.1016/j.tet.2008.04.095. - DOI
-
- Herve G., Sartori G., Enderlin G., Mackenzie G., Len C. Palladium-catalysed Suzuki reaction in aqueous solvents applied to unprotected nucleosides and nucleotides. RSC Adv. 2014;4:18558–18594. doi: 10.1039/c3ra47911k. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

