Tenascin-C and carcinoma cell invasion in oral and urinary bladder cancer
- PMID: 25793577
- PMCID: PMC4422813
- DOI: 10.1080/19336918.2015.1005463
Tenascin-C and carcinoma cell invasion in oral and urinary bladder cancer
Abstract
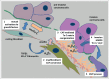
Carcinoma invasion is a complex process regulated by genetic and epigenetic factors as well. A relevant supportive condition for cancer cell migration is the reorganization of the extracellular matrix (ECM), which is realized in an orchestrated multicellular manner including carcinoma cells and stromal fibroblasts. An important key player in the process of ECM reorganization is Tenascin-C (Tn-C). The molecule occurs as different isoforms generated by alternative splicing and de novo glycosylation. Large variants of Tn-C are abundantly re-expressed in the invasive front of many carcinoma types. A special role for initiating migration and accompanied epithelial to mesenchymal transition has been suggested. Here, we review the current knowledge concerning the tumor biological importance of Tn-C, the synthesis and alternative splicing during the invasive process in general, and give an overview on the impact of Tn-C in urothelial carcinoma of the urinary bladder (UBC) and oral squamous cell carcinoma (OSCC).
Keywords: 3D, 3 dimensional; BM, basement membane; CAF, cancer associated fibroblast; ECM reorganization; ECM, extracellular matrix; EMT, epithelial – mesenchymal transition; FGF2, fibroblast growth factor 2; FNIII, fibronectin type III like repeats; Fn, fibronectin; Ln, laminin; Lnγ2, laminin gamma 2 chain; MMP, matrix metalloproteinase; OSCC, oral squamous cell carcinoma; PDGF, platelet derived growth factor; RNA, ribonucleic acid; TGFβ1, transforming growth factor beta 1; TPA, tetradecanoylphorbol acetate; Tn-C, tenascin-C; UBC, urothelial carcinoma of the urinary bladder; alternative splicing; carcinoma invasion; hnRNPs, heterogeneous nuclear ribonucleoproteins; mRNA, messenger RNA; oncFn, oncofetal fibronectin; oncTn-C, oncofetal tenascin-C; oral squamous cell carcinoma; tenascin-C; urothelial carcinoma of the urinary bladder.
Figures

References
-
- Madar S, Goldstein I, Rotter V. ‘Cancer associated fibroblasts’–more than meets the eye. Trends Mol Med 2013; 19:447-53; PMID:23769623; http://dx.doi.org/ 10.1016/j.molmed.2013.05.004 - DOI - PubMed
-
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014; 15:178-96; PMID:24556840; http://dx.doi.org/ 10.1038/nrm3758 - DOI - PMC - PubMed
-
- Steinestel K, Eder S, Schrader AJ, Steinestel J. Clinical significance of epithelial-mesenchymal transition. Clin Trans Med 2014; 3:17; PMID:25050175; http://dx.doi.org/ 10.1186/2001-1326-3-17 - DOI - PMC - PubMed
-
- Bourdon MA, Wikstrand CJ, Furthmayr H, Matthews TJ, Bigner DD. Human glioma-mesenchymal extracellular matrix antigen defined by monoclonal antibody. Cancer Res 1983; 43:2796-805; PMID:6342760 - PubMed
-
- Chiquet M, Fambrough DM. Chick myotendinous antigen. II. A novel extracellular glycoprotein complex consisting of large disulfide-linked subunits. J Cell Biol 1984; 98:1937-46; PMID:6202699; http://dx.doi.org/ 10.1083/jcb.98.6.1937 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
