An S-(hydroxymethyl)glutathione dehydrogenase is involved in conidiation and full virulence in the rice blast fungus Magnaporthe oryzae
- PMID: 25793615
- PMCID: PMC4368689
- DOI: 10.1371/journal.pone.0120627
An S-(hydroxymethyl)glutathione dehydrogenase is involved in conidiation and full virulence in the rice blast fungus Magnaporthe oryzae
Abstract
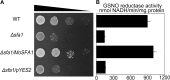
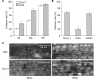
Magnaporthe oryzae is a hemibiotrophic fungal pathogen that causes rice blast disease. A compatible interaction requires overcoming plant defense responses to initiate colonization during the early infection process. Nitric oxide (NO) plays important roles in defense responses during host-pathogen interactions. Microbes generally protect themselves against NO-induced damage by using enzymes. Here, we characterized an S-(hydroxymethyl)-glutathione dehydrogenase gene in M. oryzae, MoSFA1, the homologs of which are involved in NO metabolism by specifically catalyzing the reduction of S-nitrosoglutathione (GSNO) in yeasts and plants. As expected from the activities of S-(hydroxymethyl)glutathione dehydrogenase in formaldehyde detoxification and GSNO reduction, MoSFA1 deletion mutants were lethal in formaldehyde containing medium, sensitive to exogenous NO and exhibited a higher level of S-nitrosothiols (SNOs) than that of the wild type. Notably, the mutants showed severe reduction of conidiation and appressoria turgor pressure, as well as significantly attenuated the virulence on rice cultivar CO-39. However, the virulence of MoSFA1 deletion mutants on wounded rice leaf was not affected. An infection assay on barley leaf further revealed that MoSFA1 deletion mutants exhibited a lower infection rate, and growth of infectious hyphae of the mutants was retarded not only in primary infected cells but also in expansion from cell to cell. Furthermore, barley leaf cell infected by MoSFA1 deletion mutants exhibited a stronger accumulation of H2O2 at 24 and 36 hpi. MoSFA1 deletion mutants displayed hypersensitivity to different oxidants, reduced activities of superoxide dismutases and peroxidases, and lower glutathione content in cells, compared with the wild type. These results imply that MoSFA1-mediated NO metabolism is important in redox homeostasis in response to development and host infection of M. oryzae. Taken together, this work identifies that MoSFA1 is required for conidiation and contributes to virulence in the penetration and biotrophic phases in M. oryzae.
Conflict of interest statement
Figures







References
-
- Mur LA, Carver TL, Prats E. NO way to live; the various roles of nitric oxide in plant-pathogen interactions. J Exp Bot. 2006; 57(3):489–505. - PubMed
-
- Hu X, Neill SJ, Cai W, Tang Z. NO-mediated hypersensitive responses of rice suspension cultures induced by incompatible elicitor. Chinese Sci Bull. 2003; 48(4):358–363.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

