Alpha-catulin contributes to drug-resistance of melanoma by activating NF-κB and AP-1
- PMID: 25793618
- PMCID: PMC4368766
- DOI: 10.1371/journal.pone.0119402
Alpha-catulin contributes to drug-resistance of melanoma by activating NF-κB and AP-1
Abstract
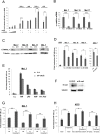
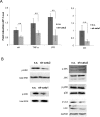
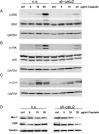
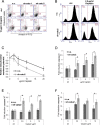
Melanoma is the most dangerous type of skin cancer accounting for 48,000 deaths worldwide each year and an average survival rate of about 6-10 months with conventional treatment. Tumor metastasis and chemoresistance of melanoma cells are reported as the main reasons for the insufficiency of currently available treatments for late stage melanoma. The cytoskeletal linker protein α-catulin (CTNNAL1) has been shown to be important in inflammation, apoptosis and cytoskeletal reorganization. Recently, we found an elevated expression of α-catulin in melanoma cells. Ectopic expression of α-catulin promoted melanoma progression and occurred concomitantly with the downregulation of E-cadherin and the upregulation of mesenchymal genes such as N-cadherin, Snail/Slug and the matrix metalloproteinases 2 and 9. In the current study we showed that α-catulin knockdown reduced NF-κB and AP-1 activity in malignant melanoma cells. Further, downregulation of α-catulin diminished ERK phosphorylation in malignant melanoma cells and sensitized them to treatment with chemotherapeutic drugs. In particular, cisplatin treatment led to decreased ERK-, JNK- and c-Jun phosphorylation in α-catulin knockdown melanoma cells, which was accompanied by enhanced apoptosis compared to control cells. Altogether, these results suggest that targeted inhibition of α-catulin may be used as a viable therapeutic strategy to chemosensitize melanoma cells to cisplatin by down-regulation of NF-κB and MAPK pathways.
Conflict of interest statement
Figures


References
-
- Chin L, Garraway LA, Fisher DE. Malignant melanoma: genetics and therapeutics in the genomic era. Genes Dev. 2006;20:2149–2182. - PubMed
-
- Hurst EA, Harbour JW, Cornelius LA. Ocular melanoma: a review and the relationship to cutaneous melanoma. Arch. Dermatol. 2003; 139, 1067–1073 - PubMed
-
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base Report on Cutaneous and Noncutaneous Melanoma. Cancer. 1998;83,8, 1664–1678 - PubMed
-
- Balch CM, Cascinelli N. The new melanoma staging system. Tumori. 2001; 87, S64–68 - PubMed
-
- Sun W, Schuchter LM. Metastatic Melanoma. Curr Treat Options Oncol 2001;2:139–202 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

