Combined hormonal versus nonhormonal versus progestin-only contraception in lactation
- PMID: 25793657
- PMCID: PMC10644229
- DOI: 10.1002/14651858.CD003988.pub2
Combined hormonal versus nonhormonal versus progestin-only contraception in lactation
Abstract
Background: Postpartum contraception improves the health of mothers and children by lengthening birth intervals. For lactating women, contraception choices are limited by concerns about hormonal effects on milk quality and quantity and passage of hormones to the infant. Ideally, the contraceptive chosen should not interfere with lactation or infant growth. Timing of contraception initiation is also important. Immediately postpartum, most women have contact with a health professional, but many do not return for follow-up contraceptive counseling. However, immediate initiation of hormonal methods may disrupt the onset of milk production.
Objectives: To determine the effects of hormonal contraceptives on lactation and infant growth
Search methods: We searched for eligible trials until 2 March 2015. Sources included the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, POPLINE, Web of Science, LILACS, ClinicalTrials.gov, and ICTRP. We also examined review articles and contacted investigators.
Selection criteria: We sought randomized controlled trials in any language that compared hormonal contraception versus another form of hormonal contraception, nonhormonal contraception, or placebo during lactation. Hormonal contraception includes combined or progestin-only oral contraceptives, injectable contraceptives, implants, and intrauterine devices.Trials had to have one of our primary outcomes: breast milk quantity or biochemical composition; lactation initiation, maintenance, or duration; infant growth; or timing of contraception initiation and effect on lactation. Secondary outcomes included contraceptive efficacy while breastfeeding and birth interval.
Data collection and analysis: For continuous variables, we calculated the mean difference (MD) with 95% confidence interval (CI). For dichotomous outcomes, we computed the Mantel-Haenszel odds ratio (OR) with 95% CI. Due to differing interventions and outcome measures, we did not aggregate the data in a meta-analysis.
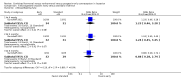
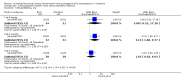
Main results: In 2014, we added seven trials for a new total of 11. Five reports were published before 1985 and six from 2005 to 2014. They included 1482 women. Four trials examined combined oral contraceptives (COCs), and three studied a levonorgestrel-releasing intrauterine system (LNG-IUS). We found two trials of progestin-only pills (POPs) and two of the etonogestrel-releasing implant. Older studies often lacked quantified results. Most trials did not report significant differences between the study arms in breastfeeding duration, breast milk composition, or infant growth. Exceptions were seen mainly in older studies with limited information.For breastfeeding duration, two of eight trials indicated a negative effect on lactation. A COC study reported a negative effect on lactation duration compared to placebo but did not quantify results. Another trial showed a lower percentage of the LNG-IUS group breastfeeding at 75 days versus the nonhormonal IUD group (reported P < 0.05) but no significant difference at one year.For breast milk volume, two older studies indicated lower volume for the COC group versus the placebo group. One trial did not quantify results. The other showed lower means (mL) for the COC group, e.g. at 16 weeks (MD -24.00, 95% CI -34.53 to -13.47) and at 24 weeks (MD -24.90, 95% CI -36.01 to -13.79). Another four trials did not report any significant difference between the study groups in milk volume or composition with two POPs, a COC, or the etonogestrel implant.Seven trials studied infant growth; one showed greater weight gain (grams) for the etonogestrel implant versus no method for six weeks (MD 426.00, 95% CI 58.94 to 793.06) but less compared with depot medroxyprogesterone acetate (DMPA) from 6 to 12 weeks (MD -271.00, 95% CI -355.10 to -186.90). The others studied POPs, COCs versus POPs, or an LNG-IUS.
Authors' conclusions: Results were not consistent across the 11 trials. The evidence was limited for any particular hormonal method. The quality of evidence was moderate overall and low for three of four placebo-controlled trials of COCs or POPs. The sensitivity analysis included six trials with moderate quality evidence and sufficient outcome data. Five trials indicated no significant difference between groups in breastfeeding duration (etonogestrel implant insertion times, COC versus POP, and LNG-IUS). For breast milk volume or composition, a COC study showed a negative effect, while an implant trial showed no significant difference. Of four trials that assessed infant growth, three indicated no significant difference between groups. One showed greater weight gain in the etonogestrel implant group versus no method but less versus DMPA.
Conflict of interest statement
Lopez LM, Grey TW, Stuebe AM, Chen M, Truitt ST, Gallo MF: none.
Figures
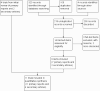

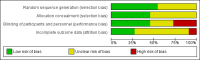











Update of
References
References to studies included in this review
Brito 2009 {published and unpublished data}
-
- Brito MB, Ferriani RA, Meijers JC, Garcia AA, Quintana SM, Silva de Sa MF, et al. Effects of the etonogestrel‐releasing contraceptive implant inserted immediately postpartum on maternal hemostasis: a randomized controlled trial. Thrombosis Research 2012;130(3):355‐60. - PubMed
-
- Brito MB, Ferriani RA, Quintana SM, Yazlle ME, Silva de Sa MF, Vieira CS. Safety of the etonogestrel‐releasing implant during the immediate postpartum period: a pilot study. Contraception 2009;80(6):519‐26. [NCT00828542] - PubMed
Dutta 2013 {published and unpublished data}
-
- Dutta DK, Dutta I. Desogestrel mini pill: is this safe in lactating mother?. Journal of the Indian Medical Association 2013; Vol. 111, issue 8:553‐5. - PubMed
Espey 2012 {published and unpublished data}
Giner Velazquez 1976 {published data only}
-
- Giner Velázquez J, Córtes Gallegos V, Sotelo López A, Bondani A. Effects of the daily administration of 0.350 mg of norethindrone on breastfeeding and milk composition [Efecto de la administracion oral diaria de 0.350 mg de noretindrona en la lactancia y en la composicion de la leche]. Ginecologia y Obstetricia de Mexico 1976;40(237):31‐9. - PubMed
Gurtcheff 2011 {published and unpublished data}
-
- Gurtcheff SE, Turok DK, Stoddard G, Murphy PA, Gibson M, Jones KP. Lactogenesis after early postpartum use of the contraceptive implant: a randomized controlled trial. Obstetrics and Gynecology 2011;117(5):1114‐21. - PubMed
Heikkilä 1982 {published data only}
-
- Heikkilä M. Puerperal insertion of a copper‐releasing and a levonorgestrel‐releasing intrauterine contraceptive device. Contraception 1982;25(6):561‐72. - PubMed
-
- Heikkilä M, Luukkainen T. Duration of breast‐feeding and development of children after insertion of a levonorgestrel‐releasing intrauterine contraceptive device. Contraception 1982;25(3):279‐92. - PubMed
Miller 1970 {published data only}
-
- Miller GH, Hughes LR. Lactation and genital involution effects of a new low‐dose oral contraceptive on breast‐feeding mothers and their infants. Obstetrics and Gynecology 1970;35(1):44‐50. - PubMed
Semm 1966 {published data only}
-
- Semm K, Dittmar FW. Post partum ovulation: inhibition and milk yield. Current Therapeutic Research 1966;8(2):48‐51. - PubMed
Shaamash 2005 {published data only}
-
- Shaamash AH, Sayed GH, Hussien MM, Shaaban MM. A comparative study of the levonorgestrel‐releasing intrauterine system Mirena versus the Copper T380A intrauterine device during lactation: breast‐feeding performance, infant growth and infant development. Contraception 2005;72(5):346‐51. - PubMed
Stuart 2014 {published and unpublished data}
-
- Stuart G. Postpartum levonorgestrel‐releasing intrauterine system and breastfeeding (PPIUD1). http://clinicaltrials.gov/show/NCT01555931 (accessed 13 August 2014).
-
- Stuart GS, Lesko C, Stuebe A, Bryant A. Breastfeeding and postpartum insertion of the levonorgestrel intrauterine system: a randomized trial. Obstetrics and Gynecology 2014;123(Suppl 1):15S.
WHO 1984 {published and unpublished data}
-
- Sas M, Gellen JJ, Dusitsin N, Tankeyoon M, Chalapati S, Crawford MA, et al. An investigation on the influence of steroidal contraceptives on milk lipid and fatty acids in Hungary and Thailand. WHO Special Programme of Research, Development and Research Training in Human Reproduction. Task Force on Oral Contraceptives. Contraception 1986;33(2):159‐78. - PubMed
-
- Tankeyoon M, Dusitain N, Chalapati S, Koetsawang S, Saibiang S, Sas M, et al. Effects of hormonal contraceptives on milk volume and infant growth. WHO Special Programme of Research, Development and Research Training in Human Reproduction. Task Force on Oral Contraceptives. Contraception 1984;30(6):505‐22. - PubMed
-
- World Health Organization (WHO) Task Force on Oral Contraceptives. Effects of hormonal contraceptives on breast milk composition and infant growth. Studies in Family Planning 1988;19(6):361‐9. - PubMed
References to studies excluded from this review
Abdel‐Aleem 1996 {published data only}
-
- Abdel‐Aleem H, Abol‐Oyoun EM, Shaaban MM, el‐Saeed M, Shoukry M, Makhlouf A, et al. The use of nomegestrol acetate subdermal contraceptive implant, Uniplant, during lactation. Contraception 1996;54:281‐6. - PubMed
Barsivala 1973 {published data only}
-
- Barsivala VM, Virkar KD. The effect of oral contraceptives on concentrations of various components of human milk. Contraception 1973;7:307‐12.
Bassol 2002 {published data only}
-
- Bassol S, Nava‐Hernandez MP, Hernandez‐Morales C, Trujillo‐Macias AM, Lopez‐Lozano MR, Recio R. Effects of levonorgestrel implant upon TSH and LH levels in male infants during lactation. International Journal of Gynaecology and Obstetrics 2002;76:273‐7. - PubMed
Betrabet 1987 {published and unpublished data}
-
- Betrabet SS, Shikary ZK, Toddywalla VS, Toddywalla SP, Patel D, Saxena BN. ICMR Task Force Study on Hormonal Contraception: transfer of norethisterone (NET) and levonorgestrel (LNG) from a single tablet into the infant's circulation through the mother's milk. Contraception 1987;35:517‐23. - PubMed
Bhatia 1987 {published data only}
-
- Bhatia S, Becker S, Kim YJ. The effect of oral contraceptive acceptance on fertility in the postpartum period. International Journal of Gynaecology and Obstetrics 1987;25(Suppl):1‐11. - PubMed
Borglin 1971 {published data only}
-
- Borglin Nils‐B, Sandholm Lars‐E. Effect of oral contraceptives on lactation. Fertility and Sterility 1971;22:39‐41. - PubMed
Chen 2011 {published data only}
-
- Chen BA, Hayes JL, Hohmann HL, Perriera LK, Reeves MF, Creinin MD. A randomized trial of postplacental compared to delayed insertion of the levonorgestrel‐releasing intrauterine device after vaginal delivery (abstract). Contraception 2009;80(2):205. [NCT00476021]
Croxatto 1982 {published and unpublished data}
-
- Croxatto HB, Diaz S, Peralta O, Juez G, Casado ME, Salvatierra AM, et al. Fertility regulation in nursing women: II. Comparative performance of progesterone implants versus placebo and copper T. American Journal of Obstetrics and Gynecology 1982;144:201‐8. - PubMed
Croxatto 1983 {published and unpublished data}
-
- Croxatto HB, Diaz S, Peralta O, Juez G, Herreros C, Casado ME, et al. Fertility regulation in nursing women: IV. Long‐term influence of a low‐dose combined oral contraceptive initiated at day 30 postpartum upon lactation and infant growth. Contraception 1983;27:13‐25. - PubMed
Diaz 1983 {published and unpublished data}
-
- Diaz S, Peralta O, Juez G, Herreros C, Casado ME, Salvatierra AM, et al. Fertility regulation in nursing women: III. Short‐term influence of low‐dose combined oral contraceptive upon lactation and infant growth. Contraception 1983;27:1‐10. - PubMed
Diaz 1985 {published and unpublished data}
-
- Diaz S, Herreros C, Juez G, Casado ME, Salvatierra AM, Miranda P, et al. Fertility regulation in nursing women: VII. Influence of Norplant levonorgestrel implants upon lactation and infant growth. Contraception 1985;32:53‐74. - PubMed
Diaz 1997 {published and unpublished data}
-
- Diaz S, Zepeda A, Maturana X, Reyes MV, Miranda P, Casado ME, et al. Fertility regulation in nursing women: IX. Contraceptive performance, duration of lactation, infant growth, and bleeding patterns during use of progesterone vaginal rings, progestin‐only pills, Norplant implants, and Copper T 380‐A intrauterine devices. Contraception 1997;56:223‐32. - PubMed
Drury 1986 {published data only}
-
- Drury PJ, Crawford MA, Ayeni O, Pinol A. Fatty acids in human milk and steroidal contraceptives in Hungary and Thailand. Progress in Lipid Research 1986;25:229‐33. - PubMed
Gellen 1984 {published data only}
-
- Gellen JJ, Sas M. Lactation and contraception: effect of low dose oral contraceptives on infant's growth during lactation. Orvosi Hetilap 1984;125:193‐6. - PubMed
Gupta 1974 {published data only}
-
- Gupta AN, Mathur VS, Garg SK. Effect of oral contraceptives on quantity and quality of milk secretion in human beings. Indian Journal of Medical Research 1974;62:964‐70. - PubMed
Hefnawi 1970 {published data only}
-
- Hefnawi F, Fawzi G, Badrawi MH, Kader MM, Bahgat MH. Attempts to select a suitable hormonal contraceptive during lactation. The VI World Congress of Ob‐Gyn; New York (NY). 1970.
Kader 1969 {published data only}
-
- Kader MM, Hay AA, El‐Safouri S, Aziz MT, El‐Din JS, Kamal I, et al. Clinical, biochemical, and experimental studies on lactation: III. Biochemical changes induced in human milk by gestagens. American Journal of Obstetrics and Gynecology 1969;105:978‐85. - PubMed
Kader 1975 {published data only}
-
- Kader M, Aziz MT, Bahgat MR, Talaat K, Osman A, Osman M. Effect of two long acting injectable progestogens on lactation in the human. Acta Biologica et Medica Germanica 1975;34:1199‐204. - PubMed
Kaern 1967 {published data only}
Kamal 1969a {published data only}
-
- Kamal I, Hefnawi F, Ghoneim M, Talaat M, Younis N, Tagui A, et al. Clinical, biochemical, and experimental studies on lactation: II. Clinical effects of gestagens on lactation. American Journal of Obstetrics and Gynecology 1969;105:324‐34. - PubMed
Kamal 1969b {published data only}
-
- Kamal I, Hefnawi F, Ghoneim M, Talaat M, Younis N, Tagui A, et al. Clinical, biochemical, and experimental studies on lactation: I. Lactation pattern in Egyptian women. American Journal of Obstetrics and Gynecology 1969;105:314‐6. - PubMed
Kamal 1970 {published data only}
-
- Kamal I, Hefnawi F, Ghoneim M, Abdallah M, Razek SA. Clinical, biochemical, and experimental studies on lactation: V. Clinical effects of steroids on the initiation of lactation. American Journal of Obstetrics and Gynecology 1970;108:655‐8. - PubMed
Karim 1971 {published data only}
Koetsawang 1972a {published data only}
-
- Koetsawang S, Bhiraleus P, Chiemprajert T. Effects of oral contraceptives on lactation. Fertility and Sterility 1972;23:24‐8. - PubMed
Koetsawang 1972b {published data only}
-
- Koetsawang S, Chiemprajert T, Kochananda P. The effects of injectable contraceptives on lactation. Clinical Proceedings I.P.P.F. Southeast Asia and Oceania Medical and Scientific Congress; Sydney (Australia). 1972;1:84‐9.
Peralta 1983 {published and unpublished data}
-
- Peralta O, Diaz S, Juez G, Herreros C, Casado ME, Salvatierra AM, et al. Fertility regulation in nursing women: V. Long‐term influence of a low‐dose combined oral contraceptive initiated at day 90 postpartum upon lactation and infant growth. Contraception 1983;27:27‐38. - PubMed
Rodrigues da Cunha 2001 {published data only}
-
- Rodrigues da Cunha AC, Dorea JG, Cantuaria AA. Intrauterine device and maternal copper metabolism during lactation. Contraception 2001;63(1):37‐9. - PubMed
Seth 1977 {published data only}
-
- Seth U, Yadava HS, Agarwal N, Laumas KR, Hingorani V. Effect of a subdermal silastic implant containing norethindrone acetate on human lactation. Contraception 1977;16:383‐98. - PubMed
Shaaban 2013 {published data only}
-
- Shaaban OM, Hassen SG, Nour SA, Kames MA, Yones EM. Emergency contraceptive pills as a backup for lactational amenorrhea method (LAM) of contraception: a randomized controlled trial. Contraception 2013;87(3):363‐9. - PubMed
Sinchai 1995 {published data only}
-
- Sinchai W, Sethavanich S, Asavpiriyanont S, Sittipiyasakul V, Sirikanchanakul R, Udomkiatsakul P, et al. Effects of a progestogen‐only pill (Exluton) and an intrauterine device (Multiload Cu250) on breastfeeding. Advances in Contraception 1995;2:143‐55. - PubMed
Toaff 1969 {published data only}
-
- Toaff R, Ashkenazi H, Schwartz A, Herzberg M. Effects of oestrogen and progestagen on the composition of human milk. Journal of Reproduction and Fertility 1969;19:475‐82. - PubMed
Were 1997 {published and unpublished data}
-
- Were EO, Nyongesa K, Nyongesa P. Randomized clinical trial to determine optimum initiation time of norgestrel‐progestin only contraception in Eldoret teaching hospital, Kenya. East African Medical Journal 1997;74:103‐7. - PubMed
Zanartu 1976 {published data only}
-
- Zanartu J, Aguilera E, Munoz G, Peliowsky H. Effect of a long‐acting contraceptive progestogen on lactation. Obstetrics and Gynecology 1976;47:174‐6. - PubMed
References to ongoing studies
Turok 2014 {published data only}
-
- Turok D. BLIS ‐ Breastfeeding Levonorgestrel IUD Study. http://clinicaltrials.gov/show/NCT01990703 (accessed 13 August 2014).
Additional references
AAP 2012
Bahamondes 2013
-
- Bahamondes L, Bahamondes MV, Modesto W, Tilley IB, Magalhães A, Silva JLPe, et al. Effect of hormonal contraceptives during breastfeeding on infant's milk ingestion and growth. Fertility and Sterility 2013; Vol. 100, issue 2:445‐50. - PubMed
Balshem 2011
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. - PubMed
Bohler 1996
-
- Bohler E, Bergstrom S. Child growth during weaning depends on whether mother is pregnant again. Journal of Tropical Pediatrics 1996;42(2):104‐9. - PubMed
Brownell 2012
CDC 2010
-
- Centers for Disease Control and Prevention. U.S. Medical Eligibility Criteria for Contraceptive Use, 2010. Appendix A. Summary of Changes to the World Health Organization Medical Eligibility Criteria for Contraceptive Use, 4th Edition, to Create the U.S. Medical Eligibility Criteria for Contraceptive Use, 2010. Morbidity and Mortality Weekly Report (MMWR) 2010;59(RR04):7‐10.
CDC 2011
-
- Centers for Disease Control and Prevention. Update to CDC's U.S. Medical Eligibility Criteria for Contraceptive Use, 2010: revised recommendations for the use of contraceptive methods during the postpartum period. Morbidity and Mortality Weekly Report (MMWR) 2011;60(26):878‐83. - PubMed
Conde‐Agudelo 2000
Erwin 1994
-
- Erwin PC. To use or not use combined hormonal oral contraceptives during lactation. Family Planning Perspectives 1994;26:26‐30,33. - PubMed
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. - PubMed
Hall 2012
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. John Wiley & Sons, Ltd.
Ip 2007
K4 Health 2014
-
- K4 Health. Lactational Amenorrhea Method Toolkit. https://www.k4health.org/toolkits/lam (accessed 20 August 2014).
Kapp 2010a
-
- Kapp N, Curtis K, Nanda K. Progestogen‐only contraceptive use among breastfeeding women: a systematic review. Contraception 2010;82(1):17‐37. - PubMed
Kapp 2010b
-
- Kapp N, Curtis KM. Combined oral contraceptive use among breastfeeding women: a systematic review. Contraception 2010;82(1):10‐6. - PubMed
Kennedy 2011
-
- Kennedy KI, Trussell J. Postpartum contraception and lactation. In: Hatcher RA, Trussell J, Nelson AL, Cates W, Kowal D, Policar MS, et al. editor(s). Contraceptive Technology. 20th Edition. New York: Ardent Media, Inc., 2011:483‐511.
Knijff 2000
-
- Knijff SCM, Goorissen EM, Velthuis‐te Wierik EJM, Korver T, Grimes DA. Summary of Contraindications to Oral Contraceptives. New York: Parthenon Publishing Group, Inc., 2000.
Kramer 2012
Pang 2007
-
- Pang WW, Hartmann PE. Initiation of human lactation: secretory differentiation and secretory activation. Journal of Mammary Gland Biology and Neoplasia 2007;12(4):211‐21. - PubMed
RamaRao 2013
-
- RamaRao S, Clark H, Merkatz R, Sussman H, Sitruk‐Ware R. Progesterone vaginal ring: introducing a contraceptive to meet the needs of breastfeeding women. Contraception 2013;88(5):591‐8. - PubMed
Raymond 2011
-
- Raymond EG. Progestin‐only pills. In: Hatcher RA, Trussell J, Nelson AL, Cates W, Kowal D, Policar MS, et al. editor(s). Contraceptive Technology. 20th Edition. New York: Ardent Media, Inc., 2011:237‐47.
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schulz 2010
Speroff 2004
-
- Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility. 7th Edition. Baltimore: Lippincott Williams and Wilkins, 2004.
Strauss 2005
-
- Strauss SE, Richardson WS, Glasziou P, Haynes RB. Evidence‐based Medicine: How to Practice and Teach EBM. Third Edition. New York: Churchill Livingstone, 2005.
Tsui 1997
-
- Tsui AO, Wasserheit JN, Haaga J, National Research Council (U.S.) Panel on Reproductive Health. Reproductive Health in Developing Countries: Expanding Dimensions, Building Solutions. Washington: National Academy Press, 1997. - PubMed
USAID 2009
-
- USAID, ACCESS‐FP. The Lactational Amenorrhea Method METHOD (LAM): A Postpartum Contraceptive Choice for Women Who Breastfeed; 2009. https://www.k4health.org/toolkits/lam/essential‐knowledge (accessed 20 August 2014).
Van der Wijden 2003
Westhoff 2012
-
- Westhoff CF. Unmet Need for Modern Contraceptive Methods. DHS Analytical Studies No. 28. Princeton, NJ: Office of Population Research, Princeton University, 2012 September.
WHO 1986
-
- World Health Organization. Protocols for the development and field testing of techniques for monitoring physical growth and psychosocial development. World Health Organization Division of Family Health and Division of Mental Health; 1986. WHO document: WHO/MCH/MNH.
WHO 1994
-
- World Health Organization. Progestogen‐only contraceptives during lactation: II. Infant development. World Health Organization, Task Force for Epidemiological Research on Reproductive Health; Special Programme of Research, Development, and Research Training in Human Reproduction. Contraception 1994;50(1):55‐68. - PubMed
WHO 2005
-
- World Health Organization. Report of a WHO Technical Consultation on Birth Spacing; 2005 Jun 13‐15; Geneva. http://www.who.int/maternal_child_adolescent/documents/birth_spacing.pdf (accessed 26 August 2014).
WHO 2009
-
- World Health Organization. Medical Eligibility Criteria for Contraceptive Use. Fourth Edition, 2009. http://www.who.int/reproductivehealth/publications/family_planning/97892.... Geneva: World Health Organization, (accessed 23 July 2014):16.
WHO 2010
-
- World Health Organization. Combined hormonal contraceptive use during the postpartum period: 2010. http://www.who.int/reproductivehealth/publications/family_planning/rhr_1... (accessed 21 August 2014).
WHO 2013
-
- World Health Organization. Statement for Collective Action for Postpartum Family Planning. http://www.who.int/reproductivehealth/publications/family_planning/statm... (accessed 10 September 2014).
References to other published versions of this review
Truitt 2003
-
- Truitt S, Fraser A, Grimes D, Gallo M, Schulz K. Hormonal contraception during lactation. Contraception 2003;68:233‐8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

