Depsides: lichen metabolites active against hepatitis C virus
- PMID: 25793970
- PMCID: PMC4368788
- DOI: 10.1371/journal.pone.0120405
Depsides: lichen metabolites active against hepatitis C virus
Abstract
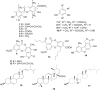
A thorough phytochemical study of Stereocaulon evolutum was conducted, for the isolation of structurally related atranorin derivatives. Indeed, pilot experiments suggested that atranorin (1), the main metabolite of this lichen, would interfere with the lifecycle of hepatitis C virus (HCV). Eight compounds, including one reported for the first time (2), were isolated and characterized. Two analogs (5, 6) were also synthesized, to enlarge the panel of atranorin-related structures. Most of these compounds were active against HCV, with a half-maximal inhibitory concentration of about 10 to 70 µM, with depsides more potent than monoaromatic phenols. The most effective inhibitors (1, 5 and 6) were then added at different steps of the HCV lifecycle. Interestingly, atranorin (1), bearing an aldehyde function at C-3, inhibited only viral entry, whereas the synthetic compounds 5 and 6, bearing a hydroxymethyl and a methyl function, respectively, at C-3 interfered with viral replication.
Conflict of interest statement
Figures



References
-
- Neyts J. Selective inhibitors of hepatitis C virus replication. Antiviral Res. 2006;71: 363–371. - PubMed
-
- Hattori M, Ma C-M, Wei Y, Dine RSE, Sato N. Survey of Anti-HIV and Anti-HCV Compounds from Natural Sources. Can Chem Trans. 2013;1: 116–140.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

