Second-hand cigarette smoke impairs bacterial phagocytosis in macrophages by modulating CFTR dependent lipid-rafts
- PMID: 25794013
- PMCID: PMC4368805
- DOI: 10.1371/journal.pone.0121200
Second-hand cigarette smoke impairs bacterial phagocytosis in macrophages by modulating CFTR dependent lipid-rafts
Abstract
Introduction: First/Second-hand cigarette-smoke (FHS/SHS) exposure weakens immune defenses inducing chronic obstructive pulmonary disease (COPD) but the underlying mechanisms are not fully understood. Hence, we evaluated if SHS induced changes in membrane/lipid-raft (m-/r)-CFTR (cystic fibrosis transmembrane conductance regulator) expression/activity is a potential mechanism for impaired bacterial phagocytosis in COPD.
Methods: RAW264.7 murine macrophages were exposed to freshly prepared CS-extract (CSE) containing culture media and/or Pseudomonas-aeruginosa-PA01-GFP for phagocytosis (fluorescence-microscopy), bacterial survival (colony-forming-units-CFU), and immunoblotting assays. The CFTR-expression/activity and lipid-rafts were modulated by transient-transfection or inhibitors/inducers. Next, mice were exposed to acute/sub-chronic-SHS or room-air (5-days/3-weeks) and infected with PA01-GFP, followed by quantification of bacterial survival by CFU-assay.
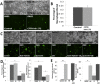
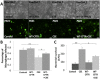
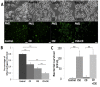
Results: We investigated the effect of CSE treatment on RAW264.7 cells infected by PA01-GFP and observed that CSE treatment significantly (p<0.01) inhibits PA01-GFP phagocytosis as compared to the controls. We also verified this in murine model, exposed to acute/sub-chronic-SHS and found significant (p<0.05, p<0.02) increase in bacterial survival in the SHS-exposed lungs as compared to the room-air controls. Next, we examined the effect of impaired CFTR ion-channel-activity on PA01-GFP infection of RAW264.7 cells using CFTR172-inhibitor and found no significant change in phagocytosis. We also similarly evaluated the effect of a CFTR corrector-potentiator compound, VRT-532, and observed no significant rescue of CSE impaired PA01-GFP phagocytosis although it significantly (p<0.05) decreases CSE induced bacterial survival. Moreover, induction of CFTR expression in macrophages significantly (p<0.03) improves CSE impaired PA01-GFP phagocytosis as compared to the control. Next, we verified the link between m-/r-CFTR expression and phagocytosis using methyl-β-cyclodextran (CD), as it is known to deplete CFTR from membrane lipid-rafts. We observed that CD treatment significantly (p<0.01) inhibits bacterial phagocytosis in RAW264.7 cells and adding CSE further impairs phagocytosis suggesting synergistic effect on CFTR dependent lipid-rafts.
Conclusion: Our data suggest that SHS impairs bacterial phagocytosis by modulating CFTR dependent lipid-rafts.
Conflict of interest statement
Figures


Similar articles
-
Augmentation of S-Nitrosoglutathione Controls Cigarette Smoke-Induced Inflammatory-Oxidative Stress and Chronic Obstructive Pulmonary Disease-Emphysema Pathogenesis by Restoring Cystic Fibrosis Transmembrane Conductance Regulator Function.Antioxid Redox Signal. 2017 Sep 1;27(7):433-451. doi: 10.1089/ars.2016.6895. Epub 2017 Feb 7. Antioxid Redox Signal. 2017. PMID: 28006950 Free PMC article.
-
Cigarette Smoke Exposure Inhibits Bacterial Killing via TFEB-Mediated Autophagy Impairment and Resulting Phagocytosis Defect.Mediators Inflamm. 2017;2017:3028082. doi: 10.1155/2017/3028082. Epub 2017 Dec 28. Mediators Inflamm. 2017. PMID: 29445254 Free PMC article.
-
Disruption of CFTR-dependent lipid rafts reduces bacterial levels and corneal disease in a murine model of Pseudomonas aeruginosa keratitis.Invest Ophthalmol Vis Sci. 2008 Mar;49(3):1000-9. doi: 10.1167/iovs.07-0993. Invest Ophthalmol Vis Sci. 2008. PMID: 18326723 Free PMC article.
-
Cigarette Smoke-Induced Acquired Dysfunction of Cystic Fibrosis Transmembrane Conductance Regulator in the Pathogenesis of Chronic Obstructive Pulmonary Disease.Oxid Med Cell Longev. 2018 Apr 23;2018:6567578. doi: 10.1155/2018/6567578. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 29849907 Free PMC article. Review.
-
CFTR dysfunction in cystic fibrosis and chronic obstructive pulmonary disease.Expert Rev Respir Med. 2018 Jun;12(6):483-492. doi: 10.1080/17476348.2018.1475235. Epub 2018 May 23. Expert Rev Respir Med. 2018. PMID: 29750581 Review.
Cited by
-
Role of mitochondria in inflammatory lung diseases.Front Pharmacol. 2024 Aug 20;15:1433961. doi: 10.3389/fphar.2024.1433961. eCollection 2024. Front Pharmacol. 2024. PMID: 39228517 Free PMC article. Review.
-
Master Autophagy Regulator Transcription Factor EB Regulates Cigarette Smoke-Induced Autophagy Impairment and Chronic Obstructive Pulmonary Disease-Emphysema Pathogenesis.Antioxid Redox Signal. 2017 Jul 20;27(3):150-167. doi: 10.1089/ars.2016.6842. Epub 2017 Feb 1. Antioxid Redox Signal. 2017. PMID: 27835930 Free PMC article.
-
Influences of environmental exposures on individuals living with cystic fibrosis.Expert Rev Respir Med. 2020 Jul;14(7):737-748. doi: 10.1080/17476348.2020.1753507. Epub 2020 Apr 26. Expert Rev Respir Med. 2020. PMID: 32264725 Free PMC article. Review.
-
CAGE sequencing reveals CFTR-dependent dysregulation of type I IFN signaling in activated cystic fibrosis macrophages.Sci Adv. 2023 May 26;9(21):eadg5128. doi: 10.1126/sciadv.adg5128. Epub 2023 May 26. Sci Adv. 2023. PMID: 37235648 Free PMC article.
-
Augmentation of S-Nitrosoglutathione Controls Cigarette Smoke-Induced Inflammatory-Oxidative Stress and Chronic Obstructive Pulmonary Disease-Emphysema Pathogenesis by Restoring Cystic Fibrosis Transmembrane Conductance Regulator Function.Antioxid Redox Signal. 2017 Sep 1;27(7):433-451. doi: 10.1089/ars.2016.6895. Epub 2017 Feb 7. Antioxid Redox Signal. 2017. PMID: 28006950 Free PMC article.
References
-
- Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006; 27: 397–412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

