Polarized Macrophages Have Distinct Roles in the Differentiation and Migration of Embryonic Spinal-cord-derived Neural Stem Cells After Grafting to Injured Sites of Spinal Cord
- PMID: 25794051
- PMCID: PMC4817764
- DOI: 10.1038/mt.2015.46
Polarized Macrophages Have Distinct Roles in the Differentiation and Migration of Embryonic Spinal-cord-derived Neural Stem Cells After Grafting to Injured Sites of Spinal Cord
Abstract
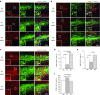
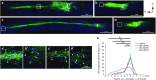
Spinal cord injury (SCI) frequently provokes serious detrimental outcomes because neuronal regeneration is limited in the central nervous system (CNS). Thus, the creation of a permissive environment for transplantation therapy with neural stem/progenitor cells (NS/PCs) is a promising strategy to replace lost neuronal cells, promote repair, and stimulate functional plasticity after SCI. Macrophages are important SCI-associated inflammatory cells and a major source of secreted factors that modify the lesion milieu. Here, we used conditional medium (CM) from bone marrow-derived M1 or M2 polarized macrophages to culture murine NS/PCs. The NS/PCs showed enhanced astrocytic versus neuronal/oligodendrocytic differentiation in the presence of M1- versus M2-CM. Similarly, cotransplantation of NS/PCs with M1 and M2 macrophages into intact or injured murine spinal cord increased the number of engrafted NS/PC-derived astrocytes and neurons/oligodendrocytes, respectively. Furthermore, when cotransplantated with M2 macrophages, the NS/PC-derived neurons integrated into the local circuitry and enhanced locomotor recovery following SCI. Interesting, engrafted M1 macrophages promoted long-distance rostral migration of NS/PC-derived cells in a chemokine (C-X-C motif) receptor 4 (CXCR4)-dependent manner, while engrafted M2 macrophages resulted in limited cell migration of NS/PC-derived cells. Altogether, these findings suggest that the cotransplantation of NS/PCs together with polarized macrophages could constitute a promising therapeutic approach for SCI repair.
Figures




Similar articles
-
Significance of remyelination by neural stem/progenitor cells transplanted into the injured spinal cord.Stem Cells. 2011 Dec;29(12):1983-94. doi: 10.1002/stem.767. Stem Cells. 2011. PMID: 22028197
-
Transplantation of galectin-1-expressing human neural stem cells into the injured spinal cord of adult common marmosets.J Neurosci Res. 2010 May 15;88(7):1394-405. doi: 10.1002/jnr.22322. J Neurosci Res. 2010. PMID: 20091712
-
Transplantation of neural stem/progenitor cells at different locations in mice with spinal cord injury.Cell Transplant. 2014;23(11):1451-64. doi: 10.3727/096368913X670967. Epub 2013 Aug 30. Cell Transplant. 2014. PMID: 23998989
-
Epigenetic regulation of neural stem cell differentiation towards spinal cord regeneration.Cell Tissue Res. 2018 Jan;371(1):189-199. doi: 10.1007/s00441-017-2656-2. Epub 2017 Jul 10. Cell Tissue Res. 2018. PMID: 28695279 Review.
-
[Transplantation of neural stem cells into spinal cord after injury].Nihon Rinsho. 2003 Mar;61(3):463-8. Nihon Rinsho. 2003. PMID: 12701174 Review. Japanese.
Cited by
-
HDAC8 Inhibition Reduces Lesional Iba-1+ Cell Infiltration after Spinal Cord Injury without Effects on Functional Recovery.Int J Mol Sci. 2020 Jun 25;21(12):4539. doi: 10.3390/ijms21124539. Int J Mol Sci. 2020. PMID: 32630606 Free PMC article.
-
Ligand-Screened Cerium-Based MOF Microcapsules Promote Nerve Regeneration via Mitochondrial Energy Supply.Adv Sci (Weinh). 2024 Feb;11(6):e2306780. doi: 10.1002/advs.202306780. Epub 2023 Nov 30. Adv Sci (Weinh). 2024. PMID: 38037294 Free PMC article.
-
Role of Aldynoglia Cells in Neuroinflammatory and Neuroimmune Responses after Spinal Cord Injury.Cells. 2021 Oct 17;10(10):2783. doi: 10.3390/cells10102783. Cells. 2021. PMID: 34685763 Free PMC article. Review.
-
Neuroprotective effect of aldose reductase knockout in a mouse model of spinal cord injury involves NF-κB pathway.Exp Brain Res. 2022 Mar;240(3):853-859. doi: 10.1007/s00221-021-06223-4. Epub 2022 Jan 23. Exp Brain Res. 2022. PMID: 35066597
-
Developmental stage of transplanted neural progenitor cells influences anatomical and functional outcomes after spinal cord injury in mice.Commun Biol. 2023 May 19;6(1):544. doi: 10.1038/s42003-023-04893-0. Commun Biol. 2023. PMID: 37208439 Free PMC article.
References
-
- Han, SS and Fischer, I (2000). Neural stem cells and gene therapy: prospects for repairing the injured spinal cord. JAMA 283: 2300–2301. - PubMed
-
- Mothe, AJ and Tator, CH (2013). Review of transplantation of neural stem/progenitor cells for spinal cord injury. Int J Dev Neurosci 31: 701–713. - PubMed
-
- Cao, QL, Zhang, YP, Howard, RM, Walters, WM, Tsoulfas, P and Whittemore, SR (2001). Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp Neurol 167: 48–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

