Pancreatic endoplasmic reticulum kinase activation promotes medulloblastoma cell migration and invasion through induction of vascular endothelial growth factor A
- PMID: 25794107
- PMCID: PMC4368580
- DOI: 10.1371/journal.pone.0120252
Pancreatic endoplasmic reticulum kinase activation promotes medulloblastoma cell migration and invasion through induction of vascular endothelial growth factor A
Abstract
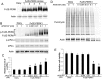
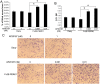
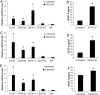
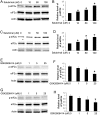
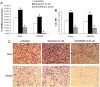
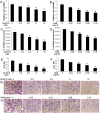
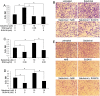
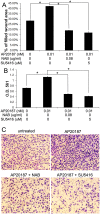
Evidence is accumulating that activation of the pancreatic endoplasmic reticulum kinase (PERK) in response to endoplasmic reticulum (ER) stress adapts tumor cells to the tumor microenvironment and enhances tumor angiogenesis by inducing vascular endothelial growth factor A (VEGF-A). Recent studies suggest that VEGF-A can act directly on certain tumor cell types in an autocrine manner, via binding to VEGF receptor 2 (VEGFR2), to promote tumor cell migration and invasion. Although several reports show that PERK activation increases VEGF-A expression in medulloblastoma, the most common solid malignancy of childhood, the role that either PERK or VEGF-A plays in medulloblastoma remains elusive. In this study, we mimicked the moderate enhancement of PERK activity observed in tumor patients using a genetic approach and a pharmacologic approach, and found that moderate activation of PERK signaling facilitated medulloblastoma cell migration and invasion and increased the production of VEGF-A. Moreover, using the VEGFR2 inhibitor SU5416 and the VEGF-A neutralizing antibody to block VEGF-A/VEGFR2 signaling, our results suggested that tumor cell-derived VEGF-A promoted medulloblastoma cell migration and invasion through VEGFR2 signaling, and that both VEGF-A and VEGFR2 were required for the promoting effects of PERK activation on medulloblastoma cell migration and invasion. Thus, these findings suggest that moderate PERK activation promotes medulloblastoma cell migration and invasion through enhancement of VEGF-A/VEGFR2 signaling.
Conflict of interest statement
Figures
Similar articles
-
A deregulated integrated stress response promotes interferon-γ-induced medulloblastoma.J Neurosci Res. 2011 Oct;89(10):1586-95. doi: 10.1002/jnr.22693. Epub 2011 Jun 17. J Neurosci Res. 2011. PMID: 21688289
-
MET Suppresses Epithelial VEGFR2 via Intracrine VEGF-induced Endoplasmic Reticulum-associated Degradation.EBioMedicine. 2015 Mar 28;2(5):406-20. doi: 10.1016/j.ebiom.2015.03.021. eCollection 2015 May. EBioMedicine. 2015. PMID: 26137585 Free PMC article.
-
Molecular controls of lymphatic VEGFR3 signaling.Arterioscler Thromb Vasc Biol. 2015 Feb;35(2):421-9. doi: 10.1161/ATVBAHA.114.304881. Epub 2014 Dec 18. Arterioscler Thromb Vasc Biol. 2015. PMID: 25524775 Free PMC article.
-
Protein kinase R(PKR)-like endoplasmic reticulum kinase (PERK) inhibitors: a patent review (2010-2015).Expert Opin Ther Pat. 2017 Jan;27(1):37-48. doi: 10.1080/13543776.2017.1238072. Epub 2016 Sep 23. Expert Opin Ther Pat. 2017. PMID: 27646439 Review.
-
Targeting mTOR as a Therapeutic Approach in Medulloblastoma.Int J Mol Sci. 2018 Jun 22;19(7):1838. doi: 10.3390/ijms19071838. Int J Mol Sci. 2018. PMID: 29932116 Free PMC article. Review.
Cited by
-
Perillyl alcohol for pediatric TP53- and RAS-mutated SHH-medulloblastoma: an in vitro and in vivo translational pre-clinical study.Childs Nerv Syst. 2021 Jul;37(7):2163-2175. doi: 10.1007/s00381-021-05115-w. Epub 2021 Apr 22. Childs Nerv Syst. 2021. PMID: 33885911
-
ER-phagy in the Occurrence and Development of Cancer.Biomedicines. 2022 Mar 18;10(3):707. doi: 10.3390/biomedicines10030707. Biomedicines. 2022. PMID: 35327508 Free PMC article. Review.
-
Unconventional Protein Secretion in Brain Tumors Biology: Enlightening the Mechanisms for Tumor Survival and Progression.Front Cell Dev Biol. 2022 Jun 15;10:907423. doi: 10.3389/fcell.2022.907423. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35784465 Free PMC article. Review.
-
Helicobacter pylori and Gastric Cancer: Adaptive Cellular Mechanisms Involved in Disease Progression.Front Microbiol. 2018 Jan 22;9:5. doi: 10.3389/fmicb.2018.00005. eCollection 2018. Front Microbiol. 2018. PMID: 29403459 Free PMC article. Review.
-
Emerging Roles of the Endoplasmic Reticulum Associated Unfolded Protein Response in Cancer Cell Migration and Invasion.Cancers (Basel). 2019 May 6;11(5):631. doi: 10.3390/cancers11050631. Cancers (Basel). 2019. PMID: 31064137 Free PMC article. Review.
References
-
- Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006; 86: 1133–1149. - PubMed
-
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8: 519–529. - PubMed
-
- Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer. 2004; 4: 966–977. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

