25 mg versus 50 mg dose of rectal diclofenac for prevention of post-ERCP pancreatitis in Japanese patients: a retrospective study
- PMID: 25795692
- PMCID: PMC4368931
- DOI: 10.1136/bmjopen-2014-006950
25 mg versus 50 mg dose of rectal diclofenac for prevention of post-ERCP pancreatitis in Japanese patients: a retrospective study
Abstract
Objectives: The aim of the present study was to assess the appropriate administration dose of non-steroidal anti-inflammation drugs to prevent pancreatitis after endoscopic retrograde cholangiopancreatography (ERCP). Importantly, the 100 mg dose of diclofenac recommended in Western countries has not been permitted in Japan.
Design: A retrospective study.
Settings: A single centre in Japan.
Participants: This study enrolled patients who underwent ERCP at the Department of Gastroenterology, Osaka Saiseikai Senri Hospital, from April 2011 through June 2013, and who received either a 25 or a 50 mg dose of rectal diclofenac after ERCP.
Primary outcome measure: The occurrence of post-ERCP pancreatitis (PEP). A multivariate regression model was used to assess the effect of the 50 mg dose (the 50 mg group) of rectal diclofenac and to compare it to the occurrence of PEP referring to the 25 mg group.
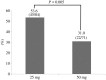
Results: A total of 155 eligible patients received either 25 mg (84 patients) or 50 mg (71 patients) doses of rectal diclofenac after ERCP to prevent PEP. The proportion of PEP was significantly lower in the 50 mg group than in the 25 mg group (15.5% (11/71) vs 33.3% (28/84), p=0.018). In a multivariate analysis, the occurrence of PEP was significantly lower in the 50 mg group than in the 25 mg group even after adjusting potential confounding factors (adjusted OR=0.27, 95% CI 0.11 to 0.70).
Conclusions: From this observation, the occurrence of PEP was significantly lower among ERCP patients with the 50 mg dose of rectal diclofenac than among those with the 25 mg dose.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
