Resimmune, an anti-CD3ε recombinant immunotoxin, induces durable remissions in patients with cutaneous T-cell lymphoma
- PMID: 25795722
- PMCID: PMC4450625
- DOI: 10.3324/haematol.2015.123711
Resimmune, an anti-CD3ε recombinant immunotoxin, induces durable remissions in patients with cutaneous T-cell lymphoma
Abstract
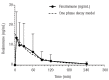
Resimmune is a second-generation recombinant immunotoxin composed of the catalytic and translocation domains of diphtheria toxin fused to two single chain antibody fragments reactive with the extracellular domain of CD3ε. We gave intravenous infusions of Resimmune 2.5 - 11.25 μg/kg over 15 minutes to 30 patients (25 with cutaneous T-cell lymphoma, 3 with peripheral T-cell lymphoma, 1 with T-cell large granular lymphocytic leukemia and 1 with T-cell prolymphocytic leukemia) in an inter-patient dose escalation trial. The most common adverse events were fever, chills, hypotension, edema, hypoalbuminemia, hypophosphatemia, and transaminasemia. Among the 25 patients with cutaneous T-cell lymphoma, there were nine responses for a response rate of 36% (95% CI, 18%-57%) including four complete remissions (16%, 95% CI, 5%-36%). The durations of the complete remissions were 72+, 72+, 60+ and 38+ months. There were five partial remissions lasting 3, 3, 3+, 6+ and 14 months. Of 17 patients with a modified skin weighted assessment tool score <50, 17 patients with stage IB/IIB, and 11 patients with both a score <50 and stage IB/IIB, nine (53%), eight (47%), and eight (73%) had responses, respectively. Further studies of Resimmune in patients with low tumor burden, stage IB-IIB cutaneous T-cell lymphoma are warranted. This trial is registered at clinicaltrials.gov as #NCT00611208.
Copyright© Ferrata Storti Foundation.
Figures


Similar articles
-
Anti-CD3 recombinant diphtheria immunotoxin therapy of cutaneous T cell lymphoma.Curr Drug Targets. 2009 Feb;10(2):104-9. doi: 10.2174/138945009787354539. Curr Drug Targets. 2009. PMID: 19199905 Review.
-
Antitumor activity of DAB389IL-2 fusion toxin in mycosis fungoides.J Am Acad Dermatol. 1998 Jul;39(1):63-73. doi: 10.1016/s0190-9622(98)70403-7. J Am Acad Dermatol. 1998. PMID: 9674399 Clinical Trial.
-
Pharmacology of anti-CD3 diphtheria immunotoxin in CD3 positive T-cell lymphoma trials.Methods Mol Biol. 2010;651:157-75. doi: 10.1007/978-1-60761-786-0_10. Methods Mol Biol. 2010. PMID: 20686966
-
Pilot study of denileukin diftitox alternate dosing regimen in patients with cutaneous peripheral T-cell lymphomas.Clin Lymphoma Myeloma Leuk. 2012 Jun;12(3):180-5. doi: 10.1016/j.clml.2012.01.011. Epub 2012 Apr 21. Clin Lymphoma Myeloma Leuk. 2012. PMID: 22521504 Clinical Trial.
-
Treatment with immunotoxin.Philos Trans R Soc Lond B Biol Sci. 2001 May 29;356(1409):681-9. doi: 10.1098/rstb.2001.0839. Philos Trans R Soc Lond B Biol Sci. 2001. PMID: 11375071 Free PMC article. Review.
Cited by
-
CD3e-immunotoxin spares CD62Llo Tregs and reshapes organ-specific T-cell composition by preferentially depleting CD3ehi T cells.Front Immunol. 2022 Oct 26;13:1011190. doi: 10.3389/fimmu.2022.1011190. eCollection 2022. Front Immunol. 2022. PMID: 36389741 Free PMC article.
-
Immunotoxins Targeting B cell Malignancy-Progress and Problems With Immunogenicity.Biomedicines. 2018 Dec 21;7(1):1. doi: 10.3390/biomedicines7010001. Biomedicines. 2018. PMID: 30577664 Free PMC article. Review.
-
Targeting the Inside of Cells with Biologicals: Toxin Routes in a Therapeutic Context.BioDrugs. 2023 Mar;37(2):181-203. doi: 10.1007/s40259-023-00580-y. Epub 2023 Feb 2. BioDrugs. 2023. PMID: 36729328 Free PMC article. Review.
-
Safe and Effective Sarcoma Therapy through Bispecific Targeting of EGFR and uPAR.Mol Cancer Ther. 2017 May;16(5):956-965. doi: 10.1158/1535-7163.MCT-16-0637. Epub 2017 Feb 13. Mol Cancer Ther. 2017. PMID: 28193671 Free PMC article.
-
Structure-Activity Relationship of the Dimeric and Oligomeric Forms of a Cytotoxic Biotherapeutic Based on Diphtheria Toxin.Biomolecules. 2022 Aug 12;12(8):1111. doi: 10.3390/biom12081111. Biomolecules. 2022. PMID: 36009005 Free PMC article.
References
-
- Imam MH, Shenoy PJ, Flowers CR, Phillips A, Lechowicz MJ. Incidence and survival patterns of cutaneous T-cell lymphomas in the United States. Leuk Lymphoma. 2013;54(4):752–759. - PubMed
-
- Jawed SI, Myskowski PL, Horwitz S, Moskowitz A, Querfeld C. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome) Part I. Diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70(2):205.e1–205.e16. - PubMed
-
- Jawed SI, Myskowski PL, Horwitz S, Moskowitz A, Querfeld C. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome) Part II. Prognosis, management, and future directions. J Am Acad Dermatol. 2014;70(2):223.e1–223.e17. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

