Fluorescence resonance energy transfer (FRET) and proximity ligation assays reveal functionally relevant homo- and heteromeric complexes among hyaluronan synthases HAS1, HAS2, and HAS3
- PMID: 25795779
- PMCID: PMC4416852
- DOI: 10.1074/jbc.M115.640581
Fluorescence resonance energy transfer (FRET) and proximity ligation assays reveal functionally relevant homo- and heteromeric complexes among hyaluronan synthases HAS1, HAS2, and HAS3
Abstract
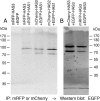
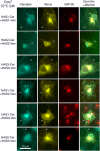
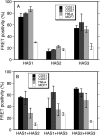
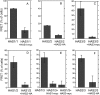
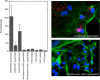
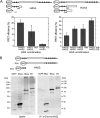
In vertebrates, hyaluronan is produced in the plasma membrane from cytosolic UDP-sugar substrates by hyaluronan synthase 1-3 (HAS1-3) isoenzymes that transfer N-acetylglucosamine (GlcNAc) and glucuronic acid (GlcUA) in alternative positions in the growing polysaccharide chain during its simultaneous extrusion into the extracellular space. It has been shown that HAS2 immunoprecipitates contain functional HAS2 homomers and also heteromers with HAS3 (Karousou, E., Kamiryo, M., Skandalis, S. S., Ruusala, A., Asteriou, T., Passi, A., Yamashita, H., Hellman, U., Heldin, C. H., and Heldin, P. (2010) The activity of hyaluronan synthase 2 is regulated by dimerization and ubiquitination. J. Biol. Chem. 285, 23647-23654). Here we have systematically screened in live cells, potential interactions among the HAS isoenzymes using fluorescence resonance energy transfer (FRET) and flow cytometric quantification. We show that all HAS isoenzymes form homomeric and also heteromeric complexes with each other. The same complexes were detected both in Golgi apparatus and plasma membrane by using FRET microscopy and the acceptor photobleaching method. Proximity ligation assays with HAS antibodies confirmed the presence of HAS1-HAS2, HAS2-HAS2, and HAS2-HAS3 complexes between endogenously expressed HASs. C-terminal deletions revealed that the enzymes interact mainly via uncharacterized N-terminal 86-amino acid domain(s), but additional binding site(s) probably exist in their C-terminal parts. Of all the homomeric complexes HAS1 had the lowest and HAS3 the highest synthetic activity. Interestingly, HAS1 transfection reduced the synthesis of hyaluronan obtained by HAS2 and HAS3, suggesting functional cooperation between the isoenzymes. These data indicate a general tendency of HAS isoenzymes to form both homomeric and heteromeric complexes with potentially important functional consequences on hyaluronan synthesis.
Keywords: Carbohydrate Biosynthesis; Fluorescence Resonance Energy Transfer (FRET); Golgi; Hyaluronan; Hyaluronan Synthase; Protein Complex; Proximity Ligation Assay.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Laurent T. C., Laurent U. B., Fraser J. R. (1996) The structure and function of hyaluronan: an overview. Immunol. Cell Biol. 74, A1–A7 - PubMed
-
- Tammi M. I., Day A. J., Turley E. A. (2002) Hyaluronan and homeostasis: a balancing act. J. Biol. Chem. 277, 4581–4584 - PubMed
-
- Bakkers J., Kramer C., Pothof J., Quaedvlieg N. E., Spaink H. P., Hammerschmidt M. (2004) Has2 is required upstream of Rac1 to govern dorsal migration of lateral cells during zebrafish gastrulation. Development 131, 525–537 - PubMed
-
- Camenisch T. D., Schroeder J. A., Bradley J., Klewer S. E., McDonald J. A. (2002) Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat. Med. 8, 850–855 - PubMed
-
- Tolg C., Hamilton S. R., Zalinska E., McCulloch L., Amin R., Akentieva N., Winnik F., Savani R., Bagli D. J., Luyt L. G., Cowman M. K., McCarthy J. B., Turley E. A. (2012) A RHAMM mimetic peptide blocks hyaluronan signaling and reduces inflammation and fibrogenesis in excisional skin wounds. Am. J. Pathol. 181, 1250–1270 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

