Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease
- PMID: 25795794
- PMCID: PMC4413801
- DOI: 10.1136/jmedgenet-2014-102797
Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease
Abstract
Background: Fabry disease results from deficient α-galactosidase A activity and globotriaosylceramide accumulation causing renal insufficiency, strokes, hypertrophic cardiomyopathy and early demise. We assessed the 10-year outcome of recombinant α-galactosidase A therapy.
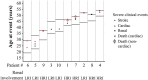
Methods: The outcomes (severe clinical events, renal function, cardiac structure) of 52/58 patients with classic Fabry disease from the phase 3 clinical trial and extension study, and the Fabry Registry were evaluated. Disease progression rates for patients with low renal involvement (LRI, n=32) or high renal involvement (HRI, n=20) at baseline were assessed.
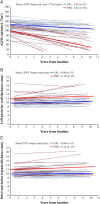
Results: 81% of patients (42/52) did not experience any severe clinical event during the treatment interval and 94% (49/52) were alive at the end of the study period. Ten patients reported a total of 16 events. Patients classified as LRI started therapy 13 years younger than HRI (mean 25 years vs 38 years). Mean slopes for estimated glomerular filtration rate for LRI and HRI were -1.89 mL/min/1.73 m(2)/year and -6.82 mL/min/1.73 m(2)/year, respectively. Overall, the mean left ventricular posterior wall thickness and interventricular septum thickness remained unchanged and normal. Patients who initiated treatment at age ≥ 40 years exhibited significant increase in left ventricular posterior wall thickness and interventricular septum thickness. Mean plasma globotriaosylceramide normalised within 6 months.
Conclusions: This 10-year study documents the effectiveness of agalsidase beta (1 mg/kg/2 weeks) in patients with Fabry disease. Most patients remained alive and event-free. Patients who initiated treatment at a younger age and with less kidney involvement benefited the most from therapy. Patients who initiated treatment at older ages and/or had advanced renal disease experienced disease progression.
Trial registration: ClinicalTrials.gov NCT00074971 NCT00196742.
Keywords: Genetics; Metabolic disorders.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures

References
-
- Schiffmann R, Warnock DG, Banikazemi M, Bultas J, Linthorst GE, Packman S, Sorensen SA, Wilcox WR, Desnick RJ. Fabry disease: progression of nephropathy, and prevalence of cardiac and cerebrovascular events before enzyme replacement therapy. Nephrol Dial Transplant 2009;24:2102–11. 10.1093/ndt/gfp031 - DOI - PMC - PubMed
-
- Colombi A, Kostyal A, Bracher R, Gloor F, Mazzi R, Tholen H. Angiokeratoma corporis diffusum—Fabry's disease. Helv Med Acta 1967;34:67–83. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
