Cell layer-specific distribution of transiently expressed barley ESCRT-III component HvVPS60 in developing barley endosperm
- PMID: 25796522
- PMCID: PMC4712231
- DOI: 10.1007/s00709-015-0798-1
Cell layer-specific distribution of transiently expressed barley ESCRT-III component HvVPS60 in developing barley endosperm
Abstract
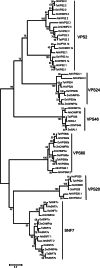
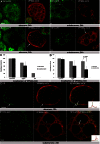
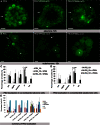
The significance of the endosomal sorting complexes required for transport (ESCRT)-III in cereal endosperm has been shown by the identification of the recessive mutant supernumerary aleurone layer1 (SAL1) in maize. ESCRT-III is indispensable in the final membrane fission step during biogenesis of multivesicular bodies (MVBs), responsible for protein sorting to vacuoles and to the cell surface. Here, we annotated barley ESCRT-III members in the (model) crop Hordeum vulgare and show that all identified members are expressed in developing barley endosperm. We used fluorescently tagged core ESCRT-III members HvSNF7a/CHMP4 and HvVPS24/CHMP3 and the associated ESCRT-III component HvVPS60a/CHMP5 for transient localization studies in barley endosperm. In vivo confocal microscopic analyses show that the localization of recombinantly expressed HvSNF7a, HvVPS24 and HvVPS60a differs within barley endosperm. Whereas HvSNF7a induces large agglomerations, HvVPS24 shows mainly cytosolic localization in aleurone and subaleurone. In contrast, HvVPS60a localizes strongly at the plasma membrane in aleurone. In subaleurone, HvVPS60a was found to a lesser extent at the plasma membrane and at vacuolar membranes. These results indicate that the steady-state association of ESCRT-III may be influenced by cell layer-specific protein deposition or trafficking and remodelling of the endomembrane system in endosperm. We show that sorting of an artificially mono-ubiquitinated Arabidopsis plasma membrane protein is inhibited by HvVPS60a in aleurone. The involvement of HvVPS60a in different cell layer-specific trafficking pathways, reflected by localization of HvVPS60a at the plasma membrane in aleurone and at the PSV membrane in subaleurone, is discussed.
Keywords: Barley; Cell layer-specific; ESCRT-III; Endosperm; Seed; VPS60.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

