Standardization of the experimental autoimmune myasthenia gravis (EAMG) model by immunization of rats with Torpedo californica acetylcholine receptors--Recommendations for methods and experimental designs
- PMID: 25796590
- PMCID: PMC4466156
- DOI: 10.1016/j.expneurol.2015.03.010
Standardization of the experimental autoimmune myasthenia gravis (EAMG) model by immunization of rats with Torpedo californica acetylcholine receptors--Recommendations for methods and experimental designs
Abstract
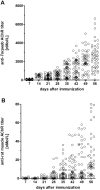
Myasthenia gravis (MG) with antibodies against the acetylcholine receptor (AChR) is characterized by a chronic, fatigable weakness of voluntary muscles. The production of autoantibodies involves the dysregulation of T cells which provide the environment for the development of autoreactive B cells. The symptoms are caused by destruction of the postsynaptic membrane and degradation of the AChR by IgG autoantibodies, predominantly of the G1 and G3 subclasses. Active immunization of animals with AChR from mammalian muscles, AChR from Torpedo or Electrophorus electric organs, and recombinant or synthetic AChR fragments generates a chronic model of MG, termed experimental autoimmune myasthenia gravis (EAMG). This model covers cellular mechanisms involved in the immune response against the AChR, e.g. antigen presentation, T cell-help and regulation, B cell selection and differentiation into plasma cells. Our aim is to define standard operation procedures and recommendations for the rat EAMG model using purified AChR from the Torpedo californica electric organ, in order to facilitate more rapid translation of preclinical proof of concept or efficacy studies into clinical trials and, ultimately, clinical practice.
Keywords: Acetylcholine receptor; Experimental autoimmune myasthenia gravis; Myasthenia gravis; Rat; Torpedo californica.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Immunization with Recombinantly Expressed LRP4 Induces Experimental Autoimmune Myasthenia Gravis in C57BL/6 Mice.Immunol Invest. 2017 Jul;46(5):490-499. doi: 10.1080/08820139.2017.1299754. Epub 2017 Apr 4. Immunol Invest. 2017. PMID: 28375749
-
Guidelines for standard preclinical experiments in the mouse model of myasthenia gravis induced by acetylcholine receptor immunization.Exp Neurol. 2015 Aug;270:11-7. doi: 10.1016/j.expneurol.2015.02.009. Epub 2015 Feb 16. Exp Neurol. 2015. PMID: 25697844
-
Protective potential of experimental autoimmune myasthenia gravis in Lewis rats by IL-10-modified dendritic cells.Neurobiol Dis. 2004 Jul;16(2):461-7. doi: 10.1016/j.nbd.2004.03.017. Neurobiol Dis. 2004. PMID: 15193302
-
Animal models of myasthenia gravis.Clin Immunol. 2000 Feb;94(2):75-87. doi: 10.1006/clim.1999.4807. Clin Immunol. 2000. PMID: 10637092 Review.
-
On the initial trigger of myasthenia gravis and suppression of the disease by antibodies against the MHC peptide region involved in the presentation of a pathogenic T-cell epitope.Crit Rev Immunol. 2001;21(1-3):1-27. Crit Rev Immunol. 2001. PMID: 11642597 Review.
Cited by
-
Engineered agrin attenuates the severity of experimental autoimmune myasthenia gravis.Muscle Nerve. 2018 May;57(5):814-820. doi: 10.1002/mus.26025. Epub 2018 Jan 8. Muscle Nerve. 2018. PMID: 29193204 Free PMC article.
-
Hypothalamic kisspeptin alleviates myasthenia gravis by regulating Th1/Th17/Treg balance through Inhibition of NF-κB signaling pathway.J Neuroinflammation. 2025 Jun 16;22(1):158. doi: 10.1186/s12974-025-03486-4. J Neuroinflammation. 2025. PMID: 40524201 Free PMC article.
-
Myasthenia Gravis Treatment: From Old Drugs to Innovative Therapies with a Glimpse into the Future.CNS Drugs. 2024 Jan;38(1):15-32. doi: 10.1007/s40263-023-01059-8. Epub 2024 Jan 11. CNS Drugs. 2024. PMID: 38212553 Review.
-
AEB-071 Ameliorates Muscle Weakness by Altering Helper T Lymphocytes in an Experimental Autoimmune Myasthenia Gravis Rat Model.Med Sci Monit. 2020 Sep 13;26:e924393. doi: 10.12659/MSM.924393. Med Sci Monit. 2020. PMID: 32920588 Free PMC article.
-
New Approaches to Targeting B Cells for Myasthenia Gravis Therapy.Front Immunol. 2020 Feb 21;11:240. doi: 10.3389/fimmu.2020.00240. eCollection 2020. Front Immunol. 2020. PMID: 32153573 Free PMC article. Review.
References
-
- Araga S, Xu L, Nakashima K, Villain M, Blalock JE. A peptide vaccine that prevents experimental autoimmune myasthenia gravis by specifically blocking T cell help. FASEB J: Off Publ Fed Am Soc Exp Biol. 2000;14:185–196. - PubMed
-
- Aricha R, Feferman T, Fuchs S, Souroujon MC. Ex vivo generated regulatory T cell s modulate experimental autoimmune myasthenia gravis. J Immunol. 2008;180:2132–2139. - PubMed
-
- Aricha R, Mizrachi K, Fuchs S, Souroujon MC. Blocking of IL-6 suppresses experimental autoimmune myasthenia gravis. J Autoimmun. 2011;36:135–141. - PubMed
-
- Asthana D, Fujii Y, Huston GE, Lindstrom J. Regulation of antibody production by helper T cell clones in experimental autoimmune myasthenia gravis is mediated by IL-4 and antigen-specific T cell factors. Clin Immunol Immunopathol. 1993;67:240–248. - PubMed
-
- Baggi F, Annoni A, Ubiali F, Milani M, Longhi R, Scaioli W, Cornelio F, Mantegazza R, Antozzi C. Breakdown of tolerance to a self-peptide of acetylcholine receptor alpha-subunit induces experimental myasthenia gravis in rats. J Immunol. 2004;172:2697–2703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

