Chimeras of channelrhodopsin-1 and -2 from Chlamydomonas reinhardtii exhibit distinctive light-induced structural changes from channelrhodopsin-2
- PMID: 25796616
- PMCID: PMC4416865
- DOI: 10.1074/jbc.M115.642256
Chimeras of channelrhodopsin-1 and -2 from Chlamydomonas reinhardtii exhibit distinctive light-induced structural changes from channelrhodopsin-2
Abstract
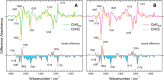
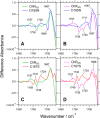
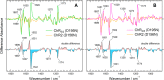
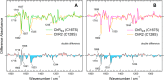
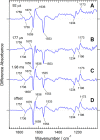
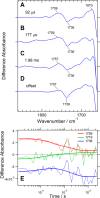
Channelrhodopsin-2 (ChR2) from the green alga Chlamydomonas reinhardtii functions as a light-gated cation channel that has been developed as an optogenetic tool to stimulate specific nerve cells in animals and control their behavior by illumination. The molecular mechanism of ChR2 has been extensively studied by a variety of spectroscopic methods, including light-induced difference Fourier transform infrared (FTIR) spectroscopy, which is sensitive to structural changes in the protein upon light activation. An atomic structure of channelrhodopsin was recently determined by x-ray crystallography using a chimera of channelrhodopsin-1 (ChR1) and ChR2. Electrophysiological studies have shown that ChR1/ChR2 chimeras are less desensitized upon continuous illumination than native ChR2, implying that there are some structural differences between ChR2 and chimeras. In this study, we applied light-induced difference FTIR spectroscopy to ChR2 and ChR1/ChR2 chimeras to determine the molecular basis underlying these functional differences. Upon continuous illumination, ChR1/ChR2 chimeras exhibited structural changes distinct from those in ChR2. In particular, the protonation state of a glutamate residue, Glu-129 (Glu-90 in ChR2 numbering), in the ChR chimeras is not changed as dramatically as in ChR2. Moreover, using mutants stabilizing particular photointermediates as well as time-resolved measurements, we identified some differences between the major photointermediates of ChR2 and ChR1/ChR2 chimeras. Taken together, our data indicate that the gating and desensitizing processes in ChR1/ChR2 chimeras are different from those in ChR2 and that these differences should be considered in the rational design of new optogenetic tools based on channelrhodopsins.
Keywords: Channel Desensitization; Channelrhodopsin; Fourier Transform IR (FTIR); Gating; Ion Channel; Photobiology; Proton Transfer; Rhodopsin.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
Proton transfer reactions in the red light-activatable channelrhodopsin variant ReaChR and their relevance for its function.J Biol Chem. 2017 Aug 25;292(34):14205-14216. doi: 10.1074/jbc.M117.779629. Epub 2017 Jun 28. J Biol Chem. 2017. PMID: 28659342 Free PMC article.
-
Channelrhodopsin-2, a directly light-gated cation-selective membrane channel.Proc Natl Acad Sci U S A. 2003 Nov 25;100(24):13940-5. doi: 10.1073/pnas.1936192100. Epub 2003 Nov 13. Proc Natl Acad Sci U S A. 2003. PMID: 14615590 Free PMC article.
-
Glu 87 of channelrhodopsin-1 causes pH-dependent color tuning and fast photocurrent inactivation.Photochem Photobiol. 2009 Mar-Apr;85(2):564-9. doi: 10.1111/j.1751-1097.2008.00519.x. Epub 2009 Jan 19. Photochem Photobiol. 2009. PMID: 19192197
-
Channelrhodopsin unchained: structure and mechanism of a light-gated cation channel.Biochim Biophys Acta. 2014 May;1837(5):626-42. doi: 10.1016/j.bbabio.2013.10.014. Epub 2013 Nov 7. Biochim Biophys Acta. 2014. PMID: 24212055 Review.
-
Novel optogenetics tool: Gt_CCR4, a light-gated cation channel with high reactivity to weak light.Biophys Rev. 2020 Apr;12(2):453-459. doi: 10.1007/s12551-020-00676-7. Epub 2020 Mar 12. Biophys Rev. 2020. PMID: 32166612 Free PMC article. Review.
Cited by
-
Molecular Dynamics of Channelrhodopsin at the Early Stages of Channel Opening.PLoS One. 2015 Jun 26;10(6):e0131094. doi: 10.1371/journal.pone.0131094. eCollection 2015. PLoS One. 2015. PMID: 26114863 Free PMC article.
-
Retinal isomerization and water-pore formation in channelrhodopsin-2.Proc Natl Acad Sci U S A. 2018 Apr 3;115(14):3557-3562. doi: 10.1073/pnas.1700091115. Epub 2018 Mar 19. Proc Natl Acad Sci U S A. 2018. PMID: 29555736 Free PMC article.
-
Gate-keeper of ion transport-a highly conserved helix-3 tryptophan in a channelrhodopsin chimera, C1C2/ChRWR.Biophys Physicobiol. 2020 Jun 9;17:59-70. doi: 10.2142/biophysico.BSJ-2020007. eCollection 2020. Biophys Physicobiol. 2020. PMID: 33173715 Free PMC article.
-
Time-resolved serial femtosecond crystallography reveals early structural changes in channelrhodopsin.Elife. 2021 Mar 23;10:e62389. doi: 10.7554/eLife.62389. Elife. 2021. PMID: 33752801 Free PMC article.
-
Spatiotemporal Control of GPR37 Signaling and Its Behavioral Effects by Optogenetics.Front Mol Neurosci. 2018 Mar 28;11:95. doi: 10.3389/fnmol.2018.00095. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29643766 Free PMC article.
References
-
- Nagel G., Ollig D., Fuhrmann M., Kateriya S., Musti A. M., Bamberg E., Hegemann P. (2002) Channelrhodopsin-1: a light-gated proton channel in green algae. Science 296, 2395–2398 - PubMed
-
- Suzuki T., Yamasaki K., Fujita S., Oda K., Iseki M., Yoshida K., Watanabe M., Daiyasu H., Toh H., Asamizu E., Tabata S., Miura K., Fukuzawa H., Nakamura S., Takahashi T. (2003) Archaeal-type rhodopsins in Chlamydomonas: model structure and intracellular localization. Biochem. Biophys. Res. Commun. 301, 711–717 - PubMed
-
- Boyden E. S., Zhang F., Bamberg E., Nagel G., Deisseroth K. (2005) Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

