Intermittent docetaxel chemotherapy is feasible for castration-resistant prostate cancer
- PMID: 25798258
- PMCID: PMC4360872
- DOI: 10.3892/mco.2014.469
Intermittent docetaxel chemotherapy is feasible for castration-resistant prostate cancer
Abstract
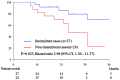
This study was conducted with the aim to investigate the feasibility of intermittent treatment with docetaxel chemotherapy for castration-resistant prostate cancer (CRPC). A total of 51 men with CRPC received docetaxel at 75 mg/m2 every 3 weeks combined with oral dexamethasone 1.0-2.0 mg/day between 2008 and 2013. The prostate-specific antigen (PSA) level was monitored every 3 weeks. Chemotherapy was suspended when the serum PSA level decreased to < 4 ng/ml, with a reduction rate of >50% from the baseline. Treatment was resumed when serum PSA increased to > 2 ng/ml, with an increase rate of >50% from the nadir. Of the 51 cases, 27 (52.9%) qualified for intermittent treatment; 17 patients received two courses of docetaxel chemotherapy and 10 received three courses. The median off-treatment interval was 266 days for the first drug holiday, 129.5 days for the second and 146.5 days for the third. The multivariate analysis indicated low baseline PSA (<median, 30.55 ng/ml; odds ratio = 0.059, P=0.010) and low Gleason score at diagnosis (≤ 7; odds ratio = 0.016, P=0.040) as significant factors for receiving intermittent therapy. The overall survival was better in intermittent cases (hazard ratio = 2.98 by log-rank test, P=0.023). During the off-treatment period, leukopenia, thrombopenia, appetite loss, diarrhea, alopecia, nail changes and fatigue subsided (0.0-11.1%), whereas sensory and/or motor neuropathy persisted in 12 of the 27 cases (44.4%). Therefore, our intermittent regimen of docetaxel chemotherapy was found to be feasible for CRPC patients, since it may reduce adverse events without compromising the oncological outcome.
Keywords: castration-resistant prostate cancer; docetaxel; intermittent therapy.
Figures
References
-
- http://www.uroweb.org/guidelines/ Nov 20th, 2014. http://www.uroweb.org/guidelines/ Access date.
-
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA. TAX 327 InvestigatorsDocetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. - DOI - PubMed
-
- Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
