YAP forms autocrine loops with the ERBB pathway to regulate ovarian cancer initiation and progression
- PMID: 25798835
- PMCID: PMC4580488
- DOI: 10.1038/onc.2015.52
YAP forms autocrine loops with the ERBB pathway to regulate ovarian cancer initiation and progression
Abstract
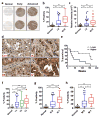
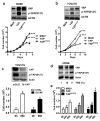
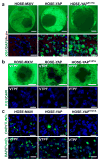
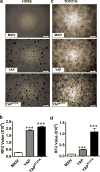
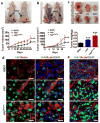
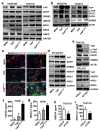
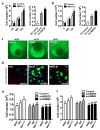
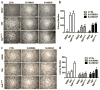
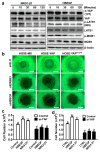
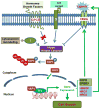
Mechanisms underlying ovarian cancer initiation and progression are unclear. Herein, we report that the Yes-associated protein (YAP), a major effector of the Hippo tumor suppressor pathway, interacts with ERBB signaling pathways to regulate the initiation and progression of ovarian cancer. Immunohistochemistry studies indicate that YAP expression is associated with poor clinical outcomes in patients. Overexpression or constitutive activation of YAP leads to transformation and tumorigenesis in human ovarian surface epithelial cells, and promotes growth of cancer cells in vivo and in vitro. YAP induces the expression of epidermal growth factor (EGF) receptors (EGFR, ERBB3) and production of EGF-like ligands (HBEGF, NRG1 and NRG2). HBEGF or NRG1, in turn, activates YAP and stimulates cancer cell growth. Knockdown of ERBB3 or HBEGF eliminates YAP effects on cell growth and transformation, whereas knockdown of YAP abrogates NRG1- and HBEGF-stimulated cell proliferation. Collectively, our study demonstrates the existence of HBEGF & NRGs/ERBBs/YAP/HBEGF & NRGs autocrine loop that controls ovarian cell tumorigenesis and cancer progression.
Conflict of interest statement
Figures
References
-
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. - PubMed
-
- Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. - PubMed
-
- Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

