Small heat-shock proteins, IbpAB, protect non-pathogenic Escherichia coli from killing by macrophage-derived reactive oxygen species
- PMID: 25798870
- PMCID: PMC4370416
- DOI: 10.1371/journal.pone.0120249
Small heat-shock proteins, IbpAB, protect non-pathogenic Escherichia coli from killing by macrophage-derived reactive oxygen species
Abstract
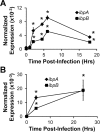
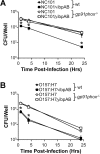
Many intracellular bacterial pathogens possess virulence factors that prevent detection and killing by macrophages. However, similar virulence factors in non-pathogenic bacteria are less well-characterized and may contribute to the pathogenesis of chronic inflammatory conditions such as Crohn's disease. We hypothesize that the small heat shock proteins IbpAB, which have previously been shown to reduce oxidative damage to proteins in vitro and be upregulated in luminal non-pathogenic Escherichia strain NC101 during experimental colitis in vivo, protect commensal E. coli from killing by macrophage-derived reactive oxygen species (ROS). Using real-time PCR, we measured ibpAB expression in commensal E. coli NC101 within wild-type (wt) and ROS-deficient (gp91phox(-/-)) macrophages and in NC101 treated with the ROS generator paraquat. We also quantified survival of NC101 and isogenic mutants in wt and gp91phox(-/-) macrophages using gentamicin protection assays. Similar assays were performed using a pathogenic E. coli strain O157:H7. We show that non-pathogenic E. coli NC101inside macrophages upregulate ibpAB within 2 hrs of phagocytosis in a ROS-dependent manner and that ibpAB protect E. coli from killing by macrophage-derived ROS. Moreover, we demonstrate that ROS-induced ibpAB expression is mediated by the small E. coli regulatory RNA, oxyS. IbpAB are not upregulated in pathogenic E. coli O157:H7 and do not affect its survival within macrophages. Together, these findings indicate that ibpAB may be novel virulence factors for certain non-pathogenic E. coli strains.
Conflict of interest statement
Figures

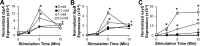


Similar articles
-
Chronic intestinal inflammation induces stress-response genes in commensal Escherichia coli.Gastroenterology. 2011 Nov;141(5):1842-51.e1-10. doi: 10.1053/j.gastro.2011.06.064. Epub 2011 Jul 2. Gastroenterology. 2011. PMID: 21726510 Free PMC article.
-
Inflammation-induced acid tolerance genes gadAB in luminal commensal Escherichia coli attenuate experimental colitis.Infect Immun. 2013 Oct;81(10):3662-71. doi: 10.1128/IAI.00355-13. Epub 2013 Jul 22. Infect Immun. 2013. PMID: 23876805 Free PMC article.
-
The global stress response regulator oxyS in an adherent-invasive Escherichia coli strain attenuates experimental colitis.Gut Microbes. 2025 Dec;17(1):2473518. doi: 10.1080/19490976.2025.2473518. Epub 2025 Mar 1. Gut Microbes. 2025. PMID: 40022675 Free PMC article.
-
Small heat shock proteins, IbpA and IbpB, are involved in resistances to heat and superoxide stresses in Escherichia coli.FEMS Microbiol Lett. 2000 Mar 15;184(2):165-71. doi: 10.1111/j.1574-6968.2000.tb09009.x. FEMS Microbiol Lett. 2000. PMID: 10713416
-
Escherichia coli O157:H7 virulence factors differentially impact cattle and bison macrophage killing capacity.Microb Pathog. 2018 May;118:251-256. doi: 10.1016/j.micpath.2018.03.045. Epub 2018 Mar 26. Microb Pathog. 2018. PMID: 29588211
Cited by
-
Legionella pneumophila Cas2 Promotes the Expression of Small Heat Shock Protein C2 That Is Required for Thermal Tolerance and Optimal Intracellular Infection.Infect Immun. 2022 Oct 20;90(10):e0036922. doi: 10.1128/iai.00369-22. Epub 2022 Sep 8. Infect Immun. 2022. PMID: 36073935 Free PMC article.
-
The Salmonella Specific, σE-Regulated, STM1250 and AgsA, Function With the sHsps IbpA and IbpB, to Counter Oxidative Stress and Survive Macrophage Killing.Front Cell Infect Microbiol. 2019 Jul 23;9:263. doi: 10.3389/fcimb.2019.00263. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31396489 Free PMC article.
-
Mechanism to control the cell lysis and the cell survival strategy in stationary phase under heat stress.Springerplus. 2015 Oct 13;4:599. doi: 10.1186/s40064-015-1415-7. eCollection 2015. Springerplus. 2015. PMID: 26543734 Free PMC article. Review.
-
Wnt5A-Mediated Actin Organization Regulates Host Response to Bacterial Pathogens and Non-Pathogens.Front Immunol. 2021 Feb 16;11:628191. doi: 10.3389/fimmu.2020.628191. eCollection 2020. Front Immunol. 2021. PMID: 33664738 Free PMC article.
References
-
- Dubreuil JD. The whole Shebang: the gastrointestinal tract, Escherichia coli enterotoxins and secretion. Curr Issues Mol Biol. 2012;14: 71–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

