Oligodendrocyte birth and death following traumatic brain injury in adult mice
- PMID: 25798924
- PMCID: PMC4370677
- DOI: 10.1371/journal.pone.0121541
Oligodendrocyte birth and death following traumatic brain injury in adult mice
Abstract
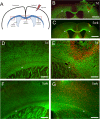
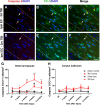
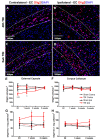
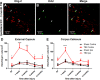
Oligodendrocytes are responsible for producing and maintaining myelin throughout the CNS. One of the pathological features observed following traumatic brain injury (TBI) is the progressive demyelination and degeneration of axons within white matter tracts. While the effect of TBI on axonal health has been well documented, there is limited information regarding the response of oligodendrocytes within these areas. The aim of this study was to characterize the response of both mature oligodendrocytes and immature proliferative oligodendrocyte lineage cells across a 3 month timecourse following TBI. A computer-controlled cortical impact model was used to produce a focal lesion in the left motor cortex of adult mice. Immunohistochemical analyses were performed at 48 hours, 7 days, 2 weeks, 5 weeks and 3 months following injury to assess the prevalence of mature CC-1+ oligodendrocyte cell death, immature Olig2+ cell proliferation and longer term survival in the corpus callosum and external capsule. Decreased CC-1 immunoreactivity was observed in white matter adjacent to the site of injury from 2 days to 2 weeks post TBI, with ongoing mature oligodendrocyte apoptosis after this time. Conversely, proliferation of Olig2+ cells was observed as early as 48 hours post TBI and significant numbers of these cells and their progeny survived and remained in the external capsule within the injured hemisphere until at least 3 months post injury. These findings demonstrate that immature oligodendrocyte lineage cells respond to TBI by replacing oligodendrocytes lost due to damage and that this process occurs for months after injury.
Conflict of interest statement
Figures

References
-
- Bramlett HM, Dietrich WD. Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol. 2002; 103: 607–614. - PubMed
-
- Johnson SC, Bigler EDB RB, Blatter DD. White Matter Atrophy, Ventricular Dilation, and Intellectual Functioning Following Traumatic Brain Injury. Neuropsych. 1994; 8: 307–315.
-
- Levin HS, Williams DH, Valastro M, Eisenberg HM, Crofford MJ, Handei SF. Corpus callosal atrophy following closed head injury: detection with magnetic resonance imaging. J. Neurosurg. 1990; 73: 77–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

