Occurrence and control of sporadic proliferation in growth arrested Swiss 3T3 feeder cells
- PMID: 25799110
- PMCID: PMC4370869
- DOI: 10.1371/journal.pone.0122056
Occurrence and control of sporadic proliferation in growth arrested Swiss 3T3 feeder cells
Abstract
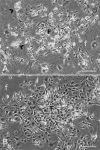
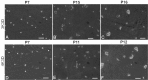
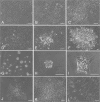
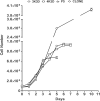
Growth arrested Swiss mouse embryonic 3T3 cells are used as feeders to support the growth of epidermal keratinocytes and several other target cells. The 3T3 cells have been extensively subcultured owing to their popularity and wide distribution in the world and, as a consequence selective inclusion of variants is a strong possibility in them. Inadvertently selected variants expressing innate resistance to mitomycin C may continue to proliferate even after treatment with such growth arresting agents. The failure of growth arrest can lead to a serious risk of proliferative feeder contamination in target cell cultures. In this study, we passaged Swiss 3T3 cells (CCL-92, ATCC) by different seeding densities and incubation periods. We tested the resultant cultures for differences in anchorage-independent growth, resumption of proliferation after mitomycin C treatment and occurrence of proliferative feeder contaminants in an epidermal keratinocyte co-culture system. The study revealed subculture dependent differential responses. The cultures of a particular subculture procedure displayed unique cell size distribution and disintegrated completely in 6 weeks following mitomycin C treatment, but their repeated subculture resulted in feeder regrowth as late as 11 weeks after the growth arrest. In contrast, mitomycin C failed to inhibit cell proliferation in cultures of the other subculture schemes and also in a clone that was established from a transformation focus of super-confluent culture. The resultant proliferative feeder cells contaminated the keratinocyte cultures. The anchorage-independent growth appeared in late passages as compared with the expression of mitomycin C resistance in earlier passages. The feeder regrowth was prevented by identifying a safe subculture protocol that discouraged the inclusion of resistant variants. We advocate routine anchorage-independent growth assay and absolute confirmation of feeder disintegration to qualify feeder batches and caution on the use of fetal bovine serum.
Conflict of interest statement
Figures





Similar articles
-
An optimization protocol for Swiss 3T3 feeder cell growth-arrest by Mitomycin C dose-to-volume derivation strategy.Cytotechnology. 2017 Apr;69(2):391-404. doi: 10.1007/s10616-017-0064-9. Epub 2017 Jan 21. Cytotechnology. 2017. PMID: 28110386 Free PMC article.
-
An evaluation of the choice of feeder cell growth arrest for the production of cultured epidermis.Burns. 2015 Dec;41(8):1788-1795. doi: 10.1016/j.burns.2015.08.011. Epub 2015 Sep 29. Burns. 2015. PMID: 26392024
-
Exposure cell number during feeder cell growth-arrest by Mitomycin C is a critical pharmacological aspect in stem cell culture system.J Pharmacol Toxicol Methods. 2016 Jul-Aug;80:68-74. doi: 10.1016/j.vascn.2016.05.006. Epub 2016 May 10. J Pharmacol Toxicol Methods. 2016. PMID: 27178105
-
Feeder Layer Cell Actions and Applications.Tissue Eng Part B Rev. 2015 Aug;21(4):345-53. doi: 10.1089/ten.TEB.2014.0547. Epub 2015 Mar 24. Tissue Eng Part B Rev. 2015. PMID: 25659081 Free PMC article. Review.
-
Derivation of Human Skin Fibroblast Lines for Feeder Cells of Human Embryonic Stem Cells.Curr Protoc Stem Cell Biol. 2016 Feb 3;36:1C.7.1-1C.7.11. doi: 10.1002/9780470151808.sc01c07s36. Curr Protoc Stem Cell Biol. 2016. PMID: 26840224 Review.
Cited by
-
An optimization protocol for Swiss 3T3 feeder cell growth-arrest by Mitomycin C dose-to-volume derivation strategy.Cytotechnology. 2017 Apr;69(2):391-404. doi: 10.1007/s10616-017-0064-9. Epub 2017 Jan 21. Cytotechnology. 2017. PMID: 28110386 Free PMC article.
-
Comparative analysis of different feeder layers with 3T3 fibroblasts for culturing rabbits limbal stem cells.Int J Ophthalmol. 2017 Jul 18;10(7):1021-1027. doi: 10.18240/ijo.2017.07.01. eCollection 2017. Int J Ophthalmol. 2017. PMID: 28730101 Free PMC article.
References
-
- Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975; 6: 331–344. - PubMed
-
- Lechner JF, Haugen A, Autrup H, McClendon IA, Trump BF, Harris CC. Clonal Growth of Epithelial Cells from Normal Adult Human Bronchus Cancer Res. 1981; 41: 2294–2304. - PubMed
-
- Navasaria HA, Sexton C, Bouvard V, Leigh IM. Growth of keratinocytes with a 3T3 feeder layer: basic techniques In: Leigh IM and Watt FM editors. The Keratinocytes Hand Book. Cambridge: Cambridge University Press; 1994. p. 5–12.
-
- Hultman CS, Brinson GM, Siltharm S, DeSerres S, Cairns BA, Peterson HD et al. Allogeneic fibroblasts used to grow cultured epidermal autografts persist in vivo and sensitize the graft recipient for accelerated second-set rejection. The Journal of Trauma. 1996; 41:51–58. - PubMed
-
- Connor DA. Mouse embryo fibroblast (MEF) feeder cell preparation. Current Protocols in Molecular Biology. 2000; 51: 23.2.1–23.2.7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

