Prostaglandin E2 and programmed cell death 1 signaling coordinately impair CTL function and survival during chronic viral infection
- PMID: 25799228
- PMCID: PMC4505619
- DOI: 10.1038/nm.3831
Prostaglandin E2 and programmed cell death 1 signaling coordinately impair CTL function and survival during chronic viral infection
Abstract
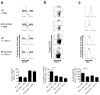
More than 10% of the world's population is chronically infected with HIV, hepatitis C virus (HCV) or hepatitis B virus (HBV), all of which can cause severe disease and death. These viruses persist in part because continuous antigenic stimulation causes the deterioration of virus-specific cytotoxic T lymphocyte (CTL) function and survival. Additionally, antiviral CTLs autonomously suppress their responses to limit immunopathology by upregulating inhibitory receptors such as programmed cell death 1 (PD-1). Identification and blockade of the pathways that induce CTL dysfunction may facilitate the clearance of chronic viral infections. We found that the prostaglandin E2 (PGE₂) receptors EP2 and EP4 were upregulated on virus-specific CTLs during chronic lymphocytic choriomeningitis virus (LCMV) infection and suppressed CTL survival and function. We show that the combined blockade of PGE₂ and PD-1 signaling was therapeutic in terms of improving viral control and augmenting the numbers of functional virus-specific CTLs. Thus, PGE₂ inhibition is both an independent candidate therapeutic target and a promising adjunct therapy to PD-1 blockade for the treatment of HIV and other chronic viral infections.
Conflict of interest statement
The authors declare competing financial interests: Jonathan Chen and Susan Kaech have applied for a patent based, in part, on the findings outlined in this paper.
Figures
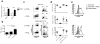

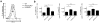


References
-
- Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408–415. - PubMed
-
- Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. - PubMed
-
- Wherry EJ. T cell exhaustion. Nat Immunol. 2011;131:492–499. - PubMed
-
- Wherry E, et al. Molecular Signature of CD8+ T Cell Exhaustion during Chronic Viral Infection. Immunity. 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

