Plasma cytokine levels fall in preterm newborn infants on nasal CPAP with early respiratory distress
- PMID: 25799377
- PMCID: PMC4370408
- DOI: 10.1371/journal.pone.0120486
Plasma cytokine levels fall in preterm newborn infants on nasal CPAP with early respiratory distress
Abstract
Introduction: Early nCPAP seems to prevent ventilator-induced lung injury in humans, although the pathophysiological mechanisms underlying this beneficial effect have not been clarified yet.
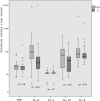
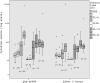
Objective: To evaluate plasma levels IL-1β, IL-6, IL-8, IL-10, and TNF-α immediately before the start of nCPAP and 2 hours later in preterm infants.
Methods: Prospective cohort including preterm infants with 28 to 35 weeks gestational age with moderate respiratory distress requiring nCPAP. Extreme preemies, newborns with malformations, congenital infections, sepsis, surfactant treatment, and receiving ventilatory support in the delivery room were excluded. Blood samples were collected right before and 2 hours after the start of nCPAP.
Results: 23 preterm infants (birth weight 1851±403 grams; GA 32.3±1.7 weeks) were treated with nCPAP. IL-1β, IL-10, TNF-α levels were similar, IL-8 levels were reduced in 18/23 preterm infants and a significant decrease in IL-6 levels was observed after 2 hours of nCPAP. All newborns whose mothers received antenatal steroids had lower cytokine levels at the onset of nCPAP than those whose mothers didn't receive it; this effect was not sustained after 2 hours of nCPAP.
Conclusion: Early use nCPAP is not associated with rising of plasma pro-inflammatory cytokines and it seems to be a less harmful respiratory strategy for preterm with moderate respiratory distress.
Conflict of interest statement
Figures
Similar articles
-
A randomized controlled trial of two nasal continuous positive airway pressure levels after extubation in preterm infants.J Pediatr. 2014 Jan;164(1):46-51. doi: 10.1016/j.jpeds.2013.08.040. Epub 2013 Oct 1. J Pediatr. 2014. PMID: 24094879 Clinical Trial.
-
Nasal intermittent positive pressure ventilation after surfactant treatment for respiratory distress syndrome in preterm infants <30 weeks' gestation: a randomized, controlled trial.J Perinatol. 2012 May;32(5):336-43. doi: 10.1038/jp.2012.1. Epub 2012 Feb 2. J Perinatol. 2012. PMID: 22301528 Clinical Trial.
-
Nasal continuous positive airway pressure (CPAP) versus bi-level nasal CPAP in preterm babies with respiratory distress syndrome: a randomised control trial.Arch Dis Child Fetal Neonatal Ed. 2010 Mar;95(2):F85-9. doi: 10.1136/adc.2009.169219. Epub 2009 Nov 29. Arch Dis Child Fetal Neonatal Ed. 2010. PMID: 19948523 Clinical Trial.
-
Weaning preterm infants from continuous positive airway pressure: evidence for best practice.World J Pediatr. 2015 Aug;11(3):212-8. doi: 10.1007/s12519-015-0022-6. Epub 2015 Apr 6. World J Pediatr. 2015. PMID: 25846068 Review.
-
CPAP and the preterm infant: lessons from the COIN trial and other studies.Early Hum Dev. 2008 Dec;84(12):791-3. doi: 10.1016/j.earlhumdev.2008.09.003. Epub 2008 Sep 19. Early Hum Dev. 2008. PMID: 18804926 Review.
Cited by
-
Comparison of Local and Systemic Inflammation During Invasive Versus Noninvasive Ventilation in Rats.J Interferon Cytokine Res. 2022 Jul;42(7):343-348. doi: 10.1089/jir.2022.0040. Epub 2022 Jun 15. J Interferon Cytokine Res. 2022. PMID: 35704907 Free PMC article.
References
-
- Naik AS, Kallapur SG, Bachurski CJ, Jobe AH, Michna J, Kramer BW, et al. Effects of ventilation with different positive end-expiratory pressures on cytokine expression in the preterm lamb lung. Am J Respir Crit Care Med. 2001; 164: 494–498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical