Clostridium perfringens type E virulence traits involved in gut colonization
- PMID: 25799452
- PMCID: PMC4370460
- DOI: 10.1371/journal.pone.0121305
Clostridium perfringens type E virulence traits involved in gut colonization
Abstract
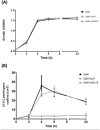
Clostridium perfringens type E disease in ruminants has been characterized by hemorrhagic enteritis or sudden death. Although type E isolates are defined by the production of alpha and iota toxin, little is known about the pathogenesis of C. perfringens type E infections. Thus far, the role of iota toxin as a virulence factor is unknown. In this report, iota toxin showed positive effects on adherence and colonization of C. perfringens type E while having negative effect on the adherence of type A cells. In-vitro and in-vivo models suggest that toxinotype E would be particularly adapted to exploit the changes induced by iota toxin in the surface of epithelial cells. In addition, type E strains produce metabolites that affected the growth of potential intra-specific competitors. These results suggest that the alteration of the enterocyte morphology induced by iota toxin concomitantly with the specific increase of type E cell adhesion and the strong intra-specific growth inhibition of other strains could be competitive traits inherent to type E isolates that improve its fitness within the bovine gut environment.
Conflict of interest statement
Figures






References
-
- McClane BA, Uzal FA, Fernandez Miyakawa ME, Lyerly DM, Wilkins TD. The enterotoxic clostridia In Dworkin M., Falkow S., Rosenberg E., Schleifer K.-H., & Stackebrandt E., Editors. The Prokaryotes: Springer US; 2006; pp. 698–752. 10.1007/0-387-30744-3 - DOI
-
- Baskerville M, Wood M, Seamer JH. Clostridium perfringens type E enterotoxaemia in rabbits. Veterinary Record. 1980; 107: 18–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

