The clinical spectrum of autoimmune congenital heart block
- PMID: 25800217
- PMCID: PMC5551504
- DOI: 10.1038/nrrheum.2015.29
The clinical spectrum of autoimmune congenital heart block
Abstract
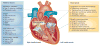
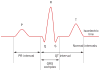
Autoimmune congenital heart block (CHB) is an immune-mediated acquired disease that is associated with the placental transference of maternal antibodies specific for Ro and La autoantigens. The disease develops in a fetal heart without anatomical abnormalities that could otherwise explain the block, and which is usually diagnosed in utero, but also at birth or within the neonatal period. Autoantibody-mediated damage of fetal conduction tissues causes inflammation and fibrosis and leads to blockage of signal conduction at the atrioventricular (AV) node. Irreversible complete AV block is the principal cardiac manifestation of CHB, although some babies might develop other severe cardiac complications, such as endocardial fibroelastosis or valvular insufficiency, even in the absence of cardiac block. In this Review, we discuss the epidemiology, classification and management of women whose pregnancies are affected by autoimmune CHB, with a particular focus on the autoantibodies associated with autoimmune CHB and how we should test for these antibodies and diagnose this disease. Without confirmed effective preventive or therapeutic strategies and further research on the aetiopathogenic mechanisms, autoimmune CHB will remain a severe life-threatening disorder.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Morquio L. Sur une maladie infantile et familiale characterisee par des modifications permanentes du pouls, des attaques syncopales et epileptiformes et la mort subite [French] Arch Med Enfants. 1901;4:467–475.
-
- Aylward RD. Congenital heart block. Br Med J. 1928;1:943.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

