Translational development of an ADAMTS-5 antibody for osteoarthritis disease modification
- PMID: 25800415
- PMCID: PMC4516626
- DOI: 10.1016/j.joca.2015.02.778
Translational development of an ADAMTS-5 antibody for osteoarthritis disease modification
Abstract
Objective/method: Aggrecanase activity, most notably ADAMTS-5, is implicated in pathogenic cartilage degradation. Selective monoclonal antibodies (mAbs) to both ADAMTS-5 and ADAMTS-4 were generated and in vitro, ex vivo and in vivo systems were utilized to assess target engagement, aggrecanase inhibition and modulation of disease-related endpoints with the intent of selecting a candidate for clinical development in osteoarthritis (OA).
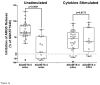
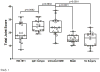
Results: Structural mapping predicts the most potent mAbs employ a unique mode of inhibition by cross-linking the catalytic and disintegrin domains. In a surgical mouse model of OA, both ADAMTS-5 and ADAMTS-4-specific mAbs penetrate cartilage following systemic administration, demonstrating access to the anticipated site of action. Structural disease modification and associated alleviation of pain-related behavior were observed with ADAMTS-5 mAb treatment. Treatment of human OA cartilage demonstrated a preferential role for ADAMTS-5 inhibition over ADAMTS-4, as measured by ARGS neoepitope release in explant cultures. ADAMTS-5 mAb activity was most evident in a subset of patient-derived tissues and suppression of ARGS neoepitope release was sustained for weeks after a single treatment in human explants and in cynomolgus monkeys, consistent with high affinity target engagement and slow ADAMTS-5 turnover.
Conclusion: This data supports a hypothesis set forth from knockout mouse studies that ADAMTS-5 is the major aggrecanase involved in cartilage degradation and provides a link between a biological pathway and pharmacology which translates to human tissues, non-human primate models and points to a target OA patient population. Therefore, a humanized ADAMTS-5-selective monoclonal antibody (GSK2394002) was progressed as a potential OA disease modifying therapeutic.
Keywords: ADAMTS-4; ADAMTS-5; Cartilage; Disease-modifying osteoarthritis drugs (DMOADs); Monoclonal antibody; Osteoarthritis.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
JL, TAL, LE, JS, JLS, RM, YX, FL, CG, CCM and CJM are current or former employees of, and are shareholders in, GlaxoSmithKline. REM, PBT and AMM declare no competing interests.
Figures














Comment in
-
ADAMTS-5 takes centre stage in new developments for aggrecanase inhibitors.Osteoarthritis Cartilage. 2015 Aug;23(8):1231-2. doi: 10.1016/j.joca.2015.05.023. Epub 2015 May 28. Osteoarthritis Cartilage. 2015. PMID: 26028137 No abstract available.
References
-
- Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. - PubMed
-
- Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

