Plasmodium vivax liver stage development and hypnozoite persistence in human liver-chimeric mice
- PMID: 25800544
- PMCID: PMC5299596
- DOI: 10.1016/j.chom.2015.02.011
Plasmodium vivax liver stage development and hypnozoite persistence in human liver-chimeric mice
Abstract
Plasmodium vivax malaria is characterized by periodic relapses of symptomatic blood stage parasite infections likely initiated by activation of dormant liver stage parasites-hypnozoites. The lack of tractable P. vivax animal models constitutes an obstacle in examining P. vivax liver stage infection and drug efficacy. To overcome this obstacle, we have used human liver-chimeric (huHep) FRG KO mice as a model for P. vivax infection. FRG KO huHep mice support P. vivax sporozoite infection, liver stage development, and hypnozoite formation. We show complete P. vivax liver stage development, including maturation into infectious exo-erythrocytic merozoites as well as the formation and persistence of hypnozoites. Prophylaxis or treatment with the antimalarial primaquine can prevent and eliminate liver stage infection, respectively. Thus, P. vivax-infected FRG KO huHep mice are a model to investigate liver stage development and dormancy and may facilitate the discovery of drugs targeting relapsing malaria.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
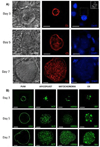
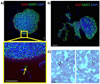

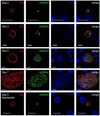
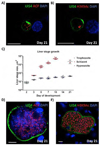
References
-
- Anstey NM, Douglas NM, Poespoprodjo JR, Price RN. Plasmodium vivax: Clinical Spectrum, Risk Factors and Pathogenesis. Advances in parasitology. 2012;80:151–201. - PubMed
-
- Collins WE, Contacos PG, Jumper JR, Smith CS, Skinner JC. Studies on human malaria in aotus monkeys. 3. Exoerythrocytic stages of the Salvador II strain of Plasmodium vivax. The Journal of parasitology. 1973;59:859–866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

