Calnexin induces expansion of antigen-specific CD4(+) T cells that confer immunity to fungal ascomycetes via conserved epitopes
- PMID: 25800545
- PMCID: PMC4484745
- DOI: 10.1016/j.chom.2015.02.009
Calnexin induces expansion of antigen-specific CD4(+) T cells that confer immunity to fungal ascomycetes via conserved epitopes
Abstract
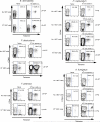
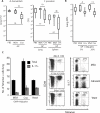
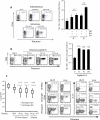
Fungal infections remain a threat due to the lack of broad-spectrum fungal vaccines and protective antigens. Recent studies showed that attenuated Blastomyces dermatitidis confers protection via T cell recognition of an unknown but conserved antigen. Using transgenic CD4(+) T cells recognizing this antigen, we identify an amino acid determinant within the chaperone calnexin that is conserved across diverse fungal ascomycetes. Calnexin, typically an ER protein, also localizes to the surface of yeast, hyphae, and spores. T cell epitope mapping unveiled a 13-residue sequence conserved across Ascomycota. Infection with divergent ascomycetes, including dimorphic fungi, opportunistic molds, and the agent causing white nose syndrome in bats, induces expansion of calnexin-specific CD4(+) T cells. Vaccine delivery of calnexin in glucan particles induces fungal antigen-specific CD4(+) T cell expansion and resistance to lethal challenge with multiple fungal pathogens. Thus, the immunogenicity and conservation of calnexin make this fungal protein a promising vaccine target.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Calnexin bridges the gap toward a pan-fungal vaccine.Cell Host Microbe. 2015 Apr 8;17(4):421-3. doi: 10.1016/j.chom.2015.03.012. Cell Host Microbe. 2015. PMID: 25856750
References
-
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nature immunology. 2007;8:639–646. - PubMed
-
- Beck O, Topp MS, Koehl U, Roilides E, Simitsopoulou M, Hanisch M, Sarfati J, Latge JP, Klingebiel T, Einsele H, et al. Generation of highly purified and functionally active human TH1 cells against Aspergillus fumigatus. Blood. 2006;107:2562–2569. - PubMed
-
- Blyth E, Clancy L, Simms R, Ma CK, Burgess J, Deo S, Byth K, Dubosq MC, Shaw PJ, Micklethwaite KP, et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood. 2013;121:3745–3758. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AI035681/AI/NIAID NIH HHS/United States
- AI093553/AI/NIAID NIH HHS/United States
- AI071118/AI/NIAID NIH HHS/United States
- R01 AI071118/AI/NIAID NIH HHS/United States
- R21 AI105816/AI/NIAID NIH HHS/United States
- R21 AI114762/AI/NIAID NIH HHS/United States
- R01 AI103760/AI/NIAID NIH HHS/United States
- R01 AI093553/AI/NIAID NIH HHS/United States
- AI040996/AI/NIAID NIH HHS/United States
- AI103760/AI/NIAID NIH HHS/United States
- R01 AI106269/AI/NIAID NIH HHS/United States
- R01 AI135005/AI/NIAID NIH HHS/United States
- R01 AI040996/AI/NIAID NIH HHS/United States
- AI105816/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

