Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma
- PMID: 25800760
- PMCID: PMC4429176
- DOI: 10.1200/JCO.2014.58.0225
Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma
Abstract
Purpose: The outcome for patients with metastatic or recurrent sarcoma remains poor. Adoptive therapy with tumor-directed T cells is an attractive therapeutic option but has never been evaluated in sarcoma.
Patients and methods: We conducted a phase I/II clinical study in which patients with recurrent/refractory human epidermal growth factor receptor 2 (HER2) -positive sarcoma received escalating doses (1 × 10(4)/m(2) to 1 × 10(8)/m(2)) of T cells expressing an HER2-specific chimeric antigen receptor with a CD28.ζ signaling domain (HER2-CAR T cells).
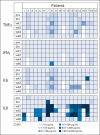
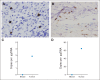
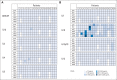
Results: We enrolled 19 patients with HER2-positive tumors (16 osteosarcomas, one Ewing sarcoma, one primitive neuroectodermal tumor, and one desmoplastic small round cell tumor). HER2-CAR T-cell infusions were well tolerated with no dose-limiting toxicity. At dose level 3 (1 × 10(5)/m(2)) and above, we detected HER2-CAR T cells 3 hours after infusion by quantitative polymerase chain reaction in 14 of 16 patients. HER2-CAR T cells persisted for at least 6 weeks in seven of the nine evaluable patients who received greater than 1 × 10(6)/m(2) HER2-CAR T cells (P = .005). HER2-CAR T cells were detected at tumor sites of two of two patients examined. Of 17 evaluable patients, four had stable disease for 12 weeks to 14 months. Three of these patients had their tumor removed, with one showing ≥ 90% necrosis. The median overall survival of all 19 infused patients was 10.3 months (range, 5.1 to 29.1 months).
Conclusion: This first evaluation of the safety and efficacy of HER2-CAR T cells in patients with cancer shows the cells can persist for 6 weeks without evident toxicities, setting the stage for studies that combine HER2-CAR T cells with other immunomodulatory approaches to enhance their expansion and persistence.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures




Comment in
-
Chimeric antigen receptor T cells for cancer immunotherapy.J Clin Oncol. 2015 May 20;33(15):1703-6. doi: 10.1200/JCO.2014.60.3449. Epub 2015 Apr 20. J Clin Oncol. 2015. PMID: 25897155 No abstract available.
References
-
- Linch M, Miah AB, Thway K, et al. Systemic treatment of soft-tissue sarcoma-gold standard and novel therapies. Nat Rev Clin Oncol. 2014;11:187–202. - PubMed
-
- Bielack SS, Carrle D, Hardes J, et al. Bone tumors in adolescents and young adults. Curr Treat Options Oncol. 2008;9:67–80. - PubMed
-
- Meyers PA. High-dose therapy with autologous stem cell rescue for pediatric sarcomas. Curr Opin Oncol. 2004;16:120–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

