Strained cyclooctyne as a molecular platform for construction of multimodal imaging probes
- PMID: 25800807
- PMCID: PMC12168436
- DOI: 10.1002/anie.201500941
Strained cyclooctyne as a molecular platform for construction of multimodal imaging probes
Abstract
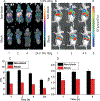
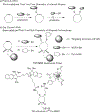
Small-molecule-based multimodal and multifunctional imaging probes play prominent roles in biomedical research and have high clinical translation ability. A novel multimodal imaging platform using base-catalyzed double addition of thiols to a strained internal alkyne such as bicyclo[6.1.0]nonyne has been established in this study, thus allowing highly selective assembly of various functional units in a protecting-group-free manner. Using this molecular platform, novel dual-modality (PET and NIRF) uPAR-targeted imaging probe: (64)Cu-CHS1 was prepared and evaluated in U87MG cells and tumor-bearing mice models. The excellent PET/NIRF imaging characteristics such as good tumor uptake (3.69%ID/g at 2 h post-injection), high tumor contrast, and specificity were achieved in the small-animal models. These attractive imaging properties make (64)Cu-CHS1 a promising probe for clinical use.
Keywords: alkynes; fluorescence; imaging agents; strained molecules; thiols.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
References
-
- Cheng L, Yang K, Li YG, Chen JH, Wang C, Shao MW, Lee ST, Liu Z, Angew. Chem. Int. Ed 2011, 50, 7385–7390; - PubMed
- Angew. Chem 2011, 123, 7523–7528;
- Cheng K, Kothapalli S, Liu H, Koh AL, Jokerst JV, Jiang H, Yang M, Li J, Levi J, Wu JC, Gambhir SS, Cheng Z, J. Am. Chem. Soc 2014, 136, 3560–3571. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

