A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury
- PMID: 25800956
- PMCID: PMC4387291
- DOI: 10.1084/jem.20141539
A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury
Abstract
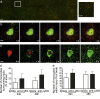
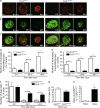
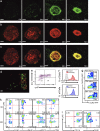
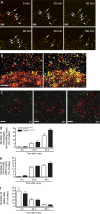
Monocytes are recruited from the blood to sites of inflammation, where they contribute to wound healing and tissue repair. There are at least two subsets of monocytes: classical or proinflammatory (CCR2(hi)CX3CR1(low)) and nonclassical, patrolling, or alternative (CCR2(low)CX3CR1(hi)) monocytes. Using spinning-disk confocal intravital microscopy and mice with fluorescent reporters for each of these subsets, we were able to track the dynamic spectrum of monocytes that enter a site of sterile hepatic injury in vivo. We observed that the CCR2(hi)CX3CR1(low) monocytes were recruited early and persisted for at least 48 h, forming a ringlike structure around the injured area. These monocytes transitioned, in situ, from CCR2(hi)Cx3CR1(low) to CX3CR1(hi)CCR2(low) within the ringlike structure and then entered the injury site. This phenotypic conversion was essential for optimal repair. These results demonstrate a local, cytokine driven reprogramming of classic, proinflammatory monocytes into nonclassical or alternative monocytes to facilitate proper wound-healing.
© 2015 Dal-Secco et al.
Figures
References
-
- Arnold L., Henry A., Poron F., Baba-Amer Y., van Rooijen N., Plonquet A., Gherardi R.K., and Chazaud B.. 2007. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204:1057–1069 10.1084/jem.20070075 - DOI - PMC - PubMed
-
- Carlin L.M., Stamatiades E.G., Auffray C., Hanna R.N., Glover L., Vizcay-Barrena G., Hedrick C.C., Cook H.T., Diebold S., and Geissmann F.. 2013. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 153:362–375 10.1016/j.cell.2013.03.010 - DOI - PMC - PubMed
-
- Engel D.R., Maurer J., Tittel A.P., Weisheit C., Cavlar T., Schumak B., Limmer A., van Rooijen N., Trautwein C., Tacke F., and Kurts C.. 2008. CCR2 mediates homeostatic and inflammatory release of Gr1(high) monocytes from the bone marrow, but is dispensable for bladder infiltration in bacterial urinary tract infection. J. Immunol. 181:5579–5586 10.4049/jimmunol.181.8.5579 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

