NMDA receptor subunits and associated signaling molecules mediating antidepressant-related effects of NMDA-GluN2B antagonism
- PMID: 25800971
- PMCID: PMC4425283
- DOI: 10.1016/j.bbr.2015.03.023
NMDA receptor subunits and associated signaling molecules mediating antidepressant-related effects of NMDA-GluN2B antagonism
Abstract
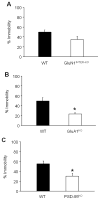
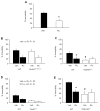
Drugs targeting the glutamate N-methyl-d-aspartate receptor (NMDAR) may be efficacious for treating mood disorders, as exemplified by the rapid antidepressant effects produced by single administration of the NMDAR antagonist ketamine. Though the precise mechanisms underlying the antidepressant-related effects of NMDAR antagonism remain unclear, recent studies implicate specific NMDAR subunits, including GluN2A and GluN2B, as well as the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR) subunit glutamate receptor interacting molecule, PSD-95. Here, integrating mutant and pharmacological in mice, we investigated the contribution of these subunits and molecules to antidepressant-related behaviors and the antidepressant-related effects of the GluN2B blocker, Ro 25-6981. We found that global deletion of GluA1 or PSD-95 reduced forced swim test (FST) immobility, mimicking the antidepressant-related effect produced by systemically administered Ro 25-6981 in C57BL/6J mice. Moreover, the FST antidepressant-like effects of systemic Ro 25-6981 were intact in mutants with global GluA1 deletion or GluN1 deletion in forebrain interneurons, but were absent in mutants constitutively lacking GluN2A or PSD-95. Next, we found that microinfusing Ro 25-6981 into the medial prefrontal cortex (mPFC), but not basolateral amygdala, of C57BL/6J mice was sufficient to produce an antidepressant-like effect. Together, these findings extend and refine current understanding of the mechanisms mediating antidepressant-like effects produced by NMDAR-GluN2B antagonists, and may inform the development of a novel class of medications for treating depression that target the GluN2B subtype of NMDAR.
Keywords: Depression; GluA1; GluN2B; Glutamate; PSD-95; Prefrontal cortex.
Copyright © 2015. Published by Elsevier B.V.
Figures

Similar articles
-
Genetic, pharmacological and lesion analyses reveal a selective role for corticohippocampal GLUN2B in a novel repeated swim stress paradigm.Neuroscience. 2011 Oct 13;193:259-68. doi: 10.1016/j.neuroscience.2011.06.015. Epub 2011 Jun 23. Neuroscience. 2011. PMID: 21704131 Free PMC article.
-
The role of GluN2A and GluN2B subunits on the effects of NMDA receptor antagonists in modeling schizophrenia and treating refractory depression.Neuropsychopharmacology. 2014 Oct;39(11):2673-80. doi: 10.1038/npp.2014.123. Epub 2014 May 29. Neuropsychopharmacology. 2014. PMID: 24871546 Free PMC article.
-
Downregulation of Egr-1 Expression Level via GluN2B Underlies the Antidepressant Effects of Ketamine in a Chronic Unpredictable Stress Animal Model of Depression.Neuroscience. 2018 Feb 21;372:38-45. doi: 10.1016/j.neuroscience.2017.12.045. Epub 2017 Dec 30. Neuroscience. 2018. PMID: 29294341
-
Two cellular hypotheses explaining the initiation of ketamine's antidepressant actions: Direct inhibition and disinhibition.Neuropharmacology. 2016 Jan;100:17-26. doi: 10.1016/j.neuropharm.2015.07.028. Epub 2015 Jul 26. Neuropharmacology. 2016. PMID: 26211972 Review.
-
Molecular and cellular mechanisms underlying the antidepressant effects of ketamine enantiomers and its metabolites.Transl Psychiatry. 2019 Nov 7;9(1):280. doi: 10.1038/s41398-019-0624-1. Transl Psychiatry. 2019. PMID: 31699965 Free PMC article. Review.
Cited by
-
Update on GPCR-based targets for the development of novel antidepressants.Mol Psychiatry. 2022 Jan;27(1):534-558. doi: 10.1038/s41380-021-01040-1. Epub 2021 Feb 15. Mol Psychiatry. 2022. PMID: 33589739 Free PMC article. Review.
-
Traxoprodil Produces Antidepressant-Like Behaviors in Chronic Unpredictable Mild Stress Mice through BDNF/ERK/CREB and AKT/FOXO/Bim Signaling Pathway.Oxid Med Cell Longev. 2023 Feb 10;2023:1131422. doi: 10.1155/2023/1131422. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 36819781 Free PMC article.
-
Ketamine: Neuroprotective or Neurotoxic?Front Neurosci. 2021 Sep 10;15:672526. doi: 10.3389/fnins.2021.672526. eCollection 2021. Front Neurosci. 2021. PMID: 34566558 Free PMC article. Review.
-
Uncoupling DAPK1 from NMDA receptor GluN2B subunit exerts rapid antidepressant-like effects.Mol Psychiatry. 2018 Mar;23(3):597-608. doi: 10.1038/mp.2017.85. Epub 2017 Apr 25. Mol Psychiatry. 2018. PMID: 28439098 Free PMC article.
-
Structural connectivity and subcellular changes after antidepressant doses of ketamine and Ro 25-6981 in the rat: an MRI and immuno-labeling study.Brain Struct Funct. 2021 Nov;226(8):2603-2616. doi: 10.1007/s00429-021-02354-0. Epub 2021 Aug 7. Brain Struct Funct. 2021. PMID: 34363521 Free PMC article.
References
-
- Yilmaz A, Schulz D, Aksoy A, Canbeyli R. Prolonged effect of an anesthetic dose of ketamine on behavioral despair. Pharmacol Biochem Behav. 2002;71:341–4. - PubMed
-
- Gigliucci V, O’Dowd G, Casey S, Egan D, Gibney S, Harkin A. Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl) 2013;228:157–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

