In vitro and in vivo efficacy of a potent opioid receptor agonist, biphalin, compared to subtype-selective opioid receptor agonists for stroke treatment
- PMID: 25801116
- PMCID: PMC4417435
- DOI: 10.1016/j.brainres.2015.03.022
In vitro and in vivo efficacy of a potent opioid receptor agonist, biphalin, compared to subtype-selective opioid receptor agonists for stroke treatment
Abstract
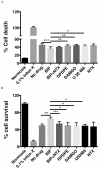
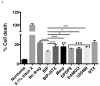
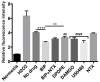
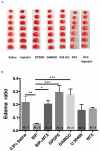
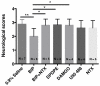
To meet the challenge of identification of new treatments for stroke, this study was designed to evaluate a potent, nonselective opioid receptor (OR) agonist, biphalin, in comparison to subtype selective OR agonists, as a potential neuroprotective drug candidate using in vitro and in vivo models of ischemic stroke. Our in vitro approach included mouse primary neuronal cells that were challenged with glutamate and hypoxic/aglycemic (H/A) conditions. We observed that 10nM biphalin, exerted a statistically significant neuroprotective effect after glutamate challenge, compared to all selective opioid agonists, according to lactate dehydrogenase (LDH) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays. Moreover, 10nM biphalin provided superior neuroprotection after H/A-reoxygenation compared to selective opioid agonists in all cases. Our in vitro investigations were supported by in vivo studies which indicate that the nonselective opioid agonist, biphalin, achieves enhanced neuroprotective potency compared to any of the selective opioid agonists, evidenced by reduced edema and infarct ratios. Reduction of edema and infarction was accompanied by neurological improvement of the animals in two independent behavioral tests. Collectively these data strongly suggest that concurrent agonist stimulation of mu, kappa and delta ORs with biphalin is neuroprotective and superior to neuroprotection by activation of any single OR subtype.
Keywords: Blood–brain barrier; Neuropeptide; Neuroprotection; Opioid receptors; Stroke.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Biphalin-A Potent Opioid Agonist-As a Panacea for Opioid System-Dependent Pathophysiological Diseases?Int J Mol Sci. 2021 Oct 21;22(21):11347. doi: 10.3390/ijms222111347. Int J Mol Sci. 2021. PMID: 34768778 Free PMC article. Review.
-
Opioid receptor agonists reduce brain edema in stroke.Brain Res. 2011 Apr 6;1383:307-16. doi: 10.1016/j.brainres.2011.01.083. Epub 2011 Jan 31. Brain Res. 2011. PMID: 21281614
-
Characterization of neuroprotective effects of biphalin, an opioid receptor agonist, in a model of focal brain ischemia.J Pharmacol Exp Ther. 2011 Nov;339(2):499-508. doi: 10.1124/jpet.111.184127. Epub 2011 Aug 19. J Pharmacol Exp Ther. 2011. PMID: 21856861 Free PMC article.
-
Enkephalin-Fentanyl Multifunctional Opioids as Potential Neuroprotectants for Ischemic Stroke Treatment.Curr Pharm Des. 2016;22(42):6459-6468. doi: 10.2174/1381612822666160720170124. Curr Pharm Des. 2016. PMID: 27510489
-
The neuroprotective role of the brain opioid system in stroke injury.Drug Discov Today. 2018 Jul;23(7):1385-1395. doi: 10.1016/j.drudis.2018.02.011. Epub 2018 Mar 1. Drug Discov Today. 2018. PMID: 29501910 Review.
Cited by
-
Blood-Brain Barrier Protection as a Therapeutic Strategy for Acute Ischemic Stroke.AAPS J. 2017 Jul;19(4):957-972. doi: 10.1208/s12248-017-0091-7. Epub 2017 May 8. AAPS J. 2017. PMID: 28484963 Review.
-
Peptides at the blood brain barrier: Knowing me knowing you.Peptides. 2015 Oct;72:50-6. doi: 10.1016/j.peptides.2015.04.020. Epub 2015 Apr 30. Peptides. 2015. PMID: 25937599 Free PMC article. Review.
-
The role of peptidase neurolysin in neuroprotection and neural repair after stroke.Neural Regen Res. 2021 Jan;16(1):21-25. doi: 10.4103/1673-5374.284904. Neural Regen Res. 2021. PMID: 32788443 Free PMC article.
-
Biphalin-A Potent Opioid Agonist-As a Panacea for Opioid System-Dependent Pathophysiological Diseases?Int J Mol Sci. 2021 Oct 21;22(21):11347. doi: 10.3390/ijms222111347. Int J Mol Sci. 2021. PMID: 34768778 Free PMC article. Review.
-
Discovery of Kynurenines Containing Oligopeptides as Potent Opioid Receptor Agonists.Biomolecules. 2020 Feb 12;10(2):284. doi: 10.3390/biom10020284. Biomolecules. 2020. PMID: 32059524 Free PMC article.
References
-
- Abbruscato TJ, Thomas SA, Hruby VJ, Davis TP. Brain and spinal cord distribution of biphalin, correlation with opioid receptor density and a mechanism of CNS entry. J. Neurochem. 1997;69(3):1236–45. - PubMed
-
- Adams HP, Jr., et al. A dose-escalation study of large doses of naloxone for treatment of patients with acute cerebral ischemia. Stroke. 1986;17:404–9. - PubMed
-
- Boutin H, et al. Differential time-course decreases in nonselective, mu-, delta-, and kappa-opioid receptors after focal cerebral ischemia in mice. Stroke. 1999;30:1271–7. discussion 1278. - PubMed
-
- Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–9. - PubMed
-
- Chao D, et al. delta-, but not mu-, opioid receptor stabilizes K(+) homeostasis by reducing Ca(2+) influx in the cortex during acute hypoxia. J Cell Physiol. 2007;212:60–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

