The perfect storm: HLA antibodies, complement, FcγRs, and endothelium in transplant rejection
- PMID: 25801125
- PMCID: PMC4424065
- DOI: 10.1016/j.molmed.2015.02.004
The perfect storm: HLA antibodies, complement, FcγRs, and endothelium in transplant rejection
Abstract
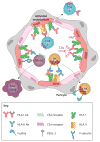
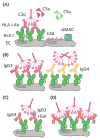
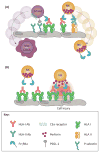
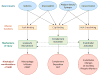
The pathophysiology of antibody-mediated rejection (AMR) in solid organ transplants is multifaceted and predominantly caused by antibodies directed against polymorphic donor human leukocyte antigens (HLAs). Despite the clearly detrimental impact of HLA antibodies (HLA-Abs) on graft function and survival, the prevention, diagnosis, and treatment of AMR remain a challenge. The histological manifestations of AMR reflect the signatures of HLA-Ab-triggered injury, specifically endothelial changes, recipient leukocytic infiltrate, and complement deposition. We review the interconnected mechanisms of HLA-Ab-mediated injury that might synergize in a 'perfect storm' of inflammation. Characterization of antibody features that are critical for effector functions may help to identify HLA-Abs that are more likely to cause rejection. We also highlight recent advances that may pave the way for new, more effective therapies.
Keywords: HLA antibodies; antibody-mediated rejection; classical complement pathway; organ transplantation.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

References
-
- Sellares J, et al. Molecular diagnosis of antibody-mediated rejection in human kidney transplants. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13:971–983. doi: 10.1111/ajt.12150. - DOI - PubMed
-
- Sis B, et al. Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9:2312–2323. doi: 10.1111/j.1600-6143.2009.02761.x. - DOI - PubMed
-
- Wu GW, et al. Asymptomatic antibody-mediated rejection after heart transplantation predicts poor outcomes. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2009;28:417–422. doi: 10.1016/j.healun.2009.01.015. - DOI - PMC - PubMed
-
- Kfoury AG, et al. A longitudinal study of the course of asymptomatic antibody-mediated rejection in heart transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2012;31:46–51. doi: 10.1016/j.healun.2011.10.009. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

