Co-transcriptional mRNP formation is coordinated within a molecular mRNP packaging station in S. cerevisiae
- PMID: 25801414
- PMCID: PMC5054900
- DOI: 10.1002/bies.201400220
Co-transcriptional mRNP formation is coordinated within a molecular mRNP packaging station in S. cerevisiae
Abstract
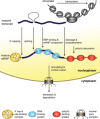
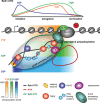
In eukaryotes, the messenger RNA (mRNA), the blueprint of a protein-coding gene, is processed and packaged into a messenger ribonucleoprotein particle (mRNP) by mRNA-binding proteins in the nucleus. The steps of mRNP formation - transcription, processing, packaging, and the orchestrated release of the export-competent mRNP from the site of transcription for nuclear mRNA export - are tightly coupled to ensure a highly efficient and regulated process. The importance of highly accurate nuclear mRNP formation is illustrated by the fact that mutations in components of this pathway lead to cellular inviability or to severe diseases in metazoans. We hypothesize that efficient mRNP formation is realized by a molecular mRNP packaging station, which is built by several recruitment platforms and coordinates the individual steps of mRNP formation.
Keywords: TREX; gene expression; mRNA processing; mRNP formation; molecular mRNP packaging station; nuclear mRNA export; transcription.
© 2015 The Authors. Bioessays published by WILEY Periodicals, Inc.
Figures
References
-
- Rondon AG, Jimeno S, Aguilera A. 2010. The interface between transcription and mRNP export: from THO to THSC/TREX‐2. Biochim Biophys Acta 1799: 533–8. - PubMed
-
- Topisirovic I, Svitkin YV, Sonenberg N, Shatkin AJ. 2011. Cap and cap‐binding proteins in the control of gene expression. Wiley Interdiscip Rev RNA 2: 277–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

