The Effects of Naltrexone on Subjective Response to Methamphetamine in a Clinical Sample: a Double-Blind, Placebo-Controlled Laboratory Study
- PMID: 25801501
- PMCID: PMC4538349
- DOI: 10.1038/npp.2015.83
The Effects of Naltrexone on Subjective Response to Methamphetamine in a Clinical Sample: a Double-Blind, Placebo-Controlled Laboratory Study
Abstract
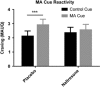
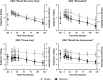
Methamphetamine (MA) use disorder is a serious psychiatric condition for which there are no FDA-approved medications. Naltrexone (NTX) is an opioid receptor antagonist with demonstrated efficacy, albeit moderate, for the treatment of alcoholism and opioid dependence. Preclinical and clinical studies suggest that NTX may be useful for the treatment of MA use disorder. To inform treatment development, we conducted a double-blind, randomized, crossover, placebo-controlled human laboratory study of NTX. Non-treatment-seeking individuals meeting DSM-IV criteria for MA abuse or dependence (n=30) completed two separate 5-day inpatient stays. During each admission, participants completed testing sessions comprised of MA cue-reactivity and intravenous MA administration (30 mg) after receiving oral NTX (50 mg) or placebo for 4 days. This study tested the hypotheses that NTX would (a) attenuate cue-induced MA craving, and (b) reduce subjective responses to MA administration. Results largely supported the study hypotheses such that (a) NTX significantly blunted cue-induced craving for MA and (b) attenuated several of the hedonic subjective effects of MA, including craving, during controlled MA administration and as compared with placebo. NTX decreased overall subjective ratings of 'crave drug,' 'stimulated,' and 'would like drug access,' decreased the the post-MA administration timecourse of 'anxious' and increased ratings of 'bad drug effects,' as compared with placebo. These findings support a potential mechanism of action by showing that NTX reduced cue-induced craving and subjective responses to MA. This is consistent with positive treatment studies of NTX for amphetamine dependence, as well as ongoing clinical trials for MA.
Figures

Similar articles
-
Naltrexone moderates the relationship between cue-induced craving and subjective response to methamphetamine in individuals with methamphetamine use disorder.Psychopharmacology (Berl). 2017 Jul;234(13):1997-2007. doi: 10.1007/s00213-017-4607-8. Epub 2017 Mar 29. Psychopharmacology (Berl). 2017. PMID: 28357460 Free PMC article.
-
Extended observation of reduced methamphetamine use with combined naltrexone plus bupropion in the ADAPT-2 trial.Addiction. 2024 Oct;119(10):1840-1845. doi: 10.1111/add.16529. Epub 2024 Jun 10. Addiction. 2024. PMID: 38856086 Clinical Trial.
-
Separate and Combined Effects of Naltrexone and Extended-Release Alprazolam on the Reinforcing, Subject-Rated, and Cardiovascular Effects of Methamphetamine.J Clin Psychopharmacol. 2016 Jun;36(3):213-21. doi: 10.1097/JCP.0000000000000488. J Clin Psychopharmacol. 2016. PMID: 27043121 Free PMC article.
-
Efficacy and safety of naltrexone for amfetamine and methamfetamine use disorder: a systematic review of randomized controlled trials.Clin Toxicol (Phila). 2019 Apr;57(4):225-233. doi: 10.1080/15563650.2018.1529317. Epub 2018 Nov 17. Clin Toxicol (Phila). 2019. PMID: 30451013
-
Intramuscular extended-release naltrexone: current evidence.Ann N Y Acad Sci. 2011 Jan;1216:144-66. doi: 10.1111/j.1749-6632.2010.05900.x. Ann N Y Acad Sci. 2011. PMID: 21272018 Review.
Cited by
-
The effects of acute oral naltrexone pretreatment on the abuse potential of intranasal methamphetamine, and the relationship between reward/punishment sensitivity and methamphetamine's effects.Behav Pharmacol. 2022 Jun 1;33(4):255-265. doi: 10.1097/FBP.0000000000000671. Epub 2022 Apr 12. Behav Pharmacol. 2022. PMID: 35438671 Free PMC article. Clinical Trial.
-
Use of Naltrexone for Patients With Stimulant Use Disorder in Malaysia: Protocol for a Retrospective Cohort Study.JMIR Res Protoc. 2025 Aug 7;14:e64101. doi: 10.2196/64101. JMIR Res Protoc. 2025. PMID: 40773739 Free PMC article.
-
A preliminary randomized clinical trial of naltrexone reduces striatal resting state functional connectivity in people with methamphetamine use disorder.Drug Alcohol Depend. 2018 Nov 1;192:186-192. doi: 10.1016/j.drugalcdep.2018.07.045. Epub 2018 Sep 21. Drug Alcohol Depend. 2018. PMID: 30266003 Free PMC article. Clinical Trial.
-
Inhibitory Effects of a Novel μ-Opioid Receptor Nonpeptide Antagonist, UD-030, on Morphine-Induced Conditioned Place Preference.Int J Mol Sci. 2023 Feb 8;24(4):3351. doi: 10.3390/ijms24043351. Int J Mol Sci. 2023. PMID: 36834763 Free PMC article.
-
A Scoping Review on Cue Reactivity in Methamphetamine Use Disorder.Int J Environ Res Public Health. 2020 Sep 7;17(18):6504. doi: 10.3390/ijerph17186504. Int J Environ Res Public Health. 2020. PMID: 32906716 Free PMC article.
References
-
- Anggadiredja K, Sakimura K, Hiranita T, Yamamoto T. Naltrexone attenuates cue- but not drug-induced methamphetamine seeking: a possible mechanism for the dissociation of primary and secondary reward. Brain Res. 2004;1021:272–276. - PubMed
-
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. - PubMed
-
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

