Haptoglobin enhances cardiac transplant rejection
- PMID: 25801896
- PMCID: PMC4426092
- DOI: 10.1161/CIRCRESAHA.116.305406
Haptoglobin enhances cardiac transplant rejection
Abstract
Rationale: Early graft inflammation enhances both acute and chronic rejection of heart transplants, but it is unclear how this inflammation is initiated.
Objective: To identify specific inflammatory modulators and determine their underlying molecular mechanisms after cardiac transplantation.
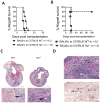
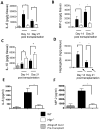
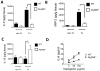
Methods and results: We used a murine heterotopic cardiac transplant model to identify inflammatory modulators of early graft inflammation. Unbiased mass spectrometric analysis of cardiac tissue before and ≤72 hours after transplantation revealed that 22 proteins including haptoglobin, a known antioxidant, are significantly upregulated in our grafts. Through the use of haptoglobin-deficient mice, we show that 80% of haptoglobin-deficient recipients treated with perioperative administration of the costimulatory blocking agent CTLA4 immunoglobulin exhibited >100-day survival of full major histocompatibility complex mismatched allografts, whereas all similarly treated wild-type recipients rejected their transplants by 21 days after transplantation. We found that haptoglobin modifies the intra-allograft inflammatory milieu by enhancing levels of the inflammatory cytokine interleukin-6 and the chemokine MIP-2 (macrophage inflammatory protein 2) but impair levels of the immunosuppressive cytokine interleukin-10. Haptoglobin also enhances dendritic cell graft recruitment and augments antidonor T-cell responses. Moreover, we confirmed that the protein is present in human cardiac allograft specimens undergoing acute graft rejection.
Conclusions: Our findings provide new insights into the mechanisms of inflammation after cardiac transplantation and suggest that, in contrast to its prior reported antioxidant function in vascular inflammation, haptoglobin is an enhancer of inflammation after cardiac transplantation. Haptoglobin may also be a key component in other sterile inflammatory conditions.
Keywords: immunology; inflammation; rejection; transplantation.
© 2015 American Heart Association, Inc.
Figures


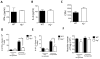

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

